Enhanced glycolysis causes extracellular acidification and activates acid-sensing ion channel 1a in hypoxic pulmonary hypertension
- PMID: 39104320
- PMCID: PMC11482464
- DOI: 10.1152/ajplung.00083.2024
Enhanced glycolysis causes extracellular acidification and activates acid-sensing ion channel 1a in hypoxic pulmonary hypertension
Abstract
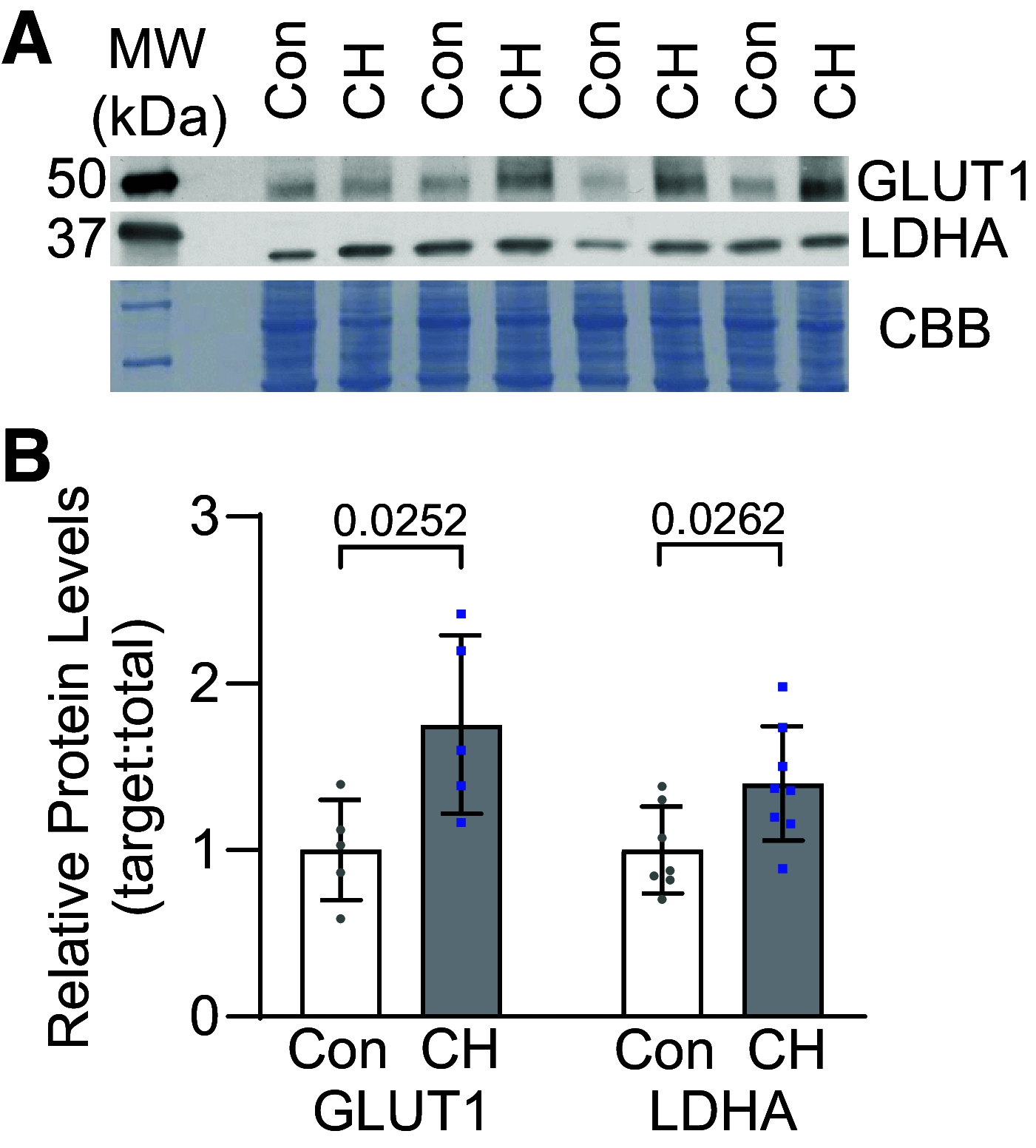
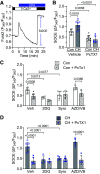
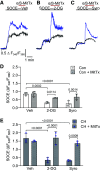
In pulmonary hypertension (PHTN), a metabolic shift to aerobic glycolysis promotes a hyperproliferative, apoptosis-resistant phenotype in pulmonary arterial smooth muscle cells (PASMCs). Enhanced glycolysis induces extracellular acidosis, which can activate proton-sensing membrane receptors and ion channels. We previously reported that activation of the proton-gated cation channel acid-sensing ion channel 1a (ASIC1a) contributes to the development of hypoxic PHTN. Therefore, we hypothesize that enhanced glycolysis and subsequent acidification of the PASMC extracellular microenvironment activate ASIC1a in hypoxic PHTN. We observed decreased oxygen consumption rate and increased extracellular acidification rate in PASMCs from chronic hypoxia (CH)-induced PHTN rats, indicating a shift to aerobic glycolysis. In addition, we found that intracellular alkalization and extracellular acidification occur in PASMCs following CH and in vitro hypoxia, which were prevented by the inhibition of glycolysis with 2-deoxy-d-glucose (2-DG). Inhibiting H+ transport/secretion through carbonic anhydrases, Na+/H+ exchanger 1, or vacuolar-type H+-ATPase did not prevent this pH shift following hypoxia. Although the putative monocarboxylate transporter 1 (MCT1) and -4 (MCT4) inhibitor syrosingopine prevented the pH shift, the specific MCT1 inhibitor AZD3965 and/or the MCT4 inhibitor VB124 were without effect, suggesting that syrosingopine targets the glycolytic pathway independent of H+ export. Furthermore, 2-DG and syrosingopine prevented enhanced ASIC1a-mediated store-operated Ca2+ entry in PASMCs from CH rats. These data suggest that multiple H+ transport mechanisms contribute to extracellular acidosis and that inhibiting glycolysis-rather than specific H+ transporters-more effectively prevents extracellular acidification and ASIC1a activation. Together, these data reveal a novel pathological relationship between glycolysis and ASIC1a activation in hypoxic PHTN.NEW & NOTEWORTHY In pulmonary hypertension, a metabolic shift to aerobic glycolysis drives a hyperproliferative, apoptosis-resistant phenotype in pulmonary arterial smooth muscle cells. We demonstrate that this enhanced glycolysis induces extracellular acidosis and activates the proton-gated ion channel, acid-sensing ion channel 1a (ASIC1a). Although multiple H+ transport/secretion mechanisms are upregulated in PHTN and likely contribute to extracellular acidosis, inhibiting glycolysis with 2-deoxy-d-glucose or syrosingopine effectively prevents extracellular acidification and ASIC1a activation, revealing a promising therapeutic avenue.
Keywords: VB124; Warburg effect; monocarboxylic acid transporters; pH regulation; vascular smooth muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg JM, Sloane BF, Johnson J, Gatenby RA, Gillies RJ. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73: 1524–1535, 2013. doi: 10.1158/0008-5472.CAN-12-2796. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

