Synthesis, characterization, and mechanistic insights into the enhanced anti-inflammatory activity of baicalin butyl ester via the PI3K-AKT pathway
- PMID: 39104394
- PMCID: PMC11298432
- DOI: 10.3389/fphar.2024.1417372
Synthesis, characterization, and mechanistic insights into the enhanced anti-inflammatory activity of baicalin butyl ester via the PI3K-AKT pathway
Abstract
Objective: To investigate the anti-inflammatory activity and mechanism of Baicalin derivative (Baicalin butyl ester, BE).
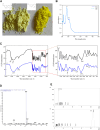
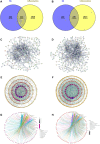
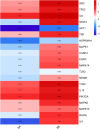
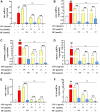
Methods: BE was synthesized and identified using UV-Vis spectroscopy, FT-IR spectroscopy, mass spectrometry (MS) and high-performance liquid chromatography (HPLC) methods. Its anti-inflammatory potential was explored by an in vitro inflammation model. Network pharmacology was employed to predict the anti-inflammatory targets of BE, construct protein-protein interaction (PPI) networks, and analysis topological features and KEGG pathway enrichment. Additionally, molecular docking was conducted to evaluate the binding affinity between BE and its core targets. qRT-PCR analysis was conducted to validate the network pharmacology results. The organizational efficiency was further evaluated through octanol-water partition coefficient and transmembrane activity analysis.
Results: UV-Vis, FT-IR, MS, and HPLC analyses confirmed the successfully synthesis of BE with a high purity of 93.75%. In vitro anti-inflammatory research showed that BE could more effectively suppress the expression of NO, COX-2, IL-6, IL-1β, and iNOS. Network pharmacology and in vitro experiments validated that BE's anti-inflammatory effects was mediated through the suppression of SRC, HSP90AA1, PIK3CA, JAK2, AKT1, and NF-κB via PI3K-AKT pathway. Molecular docking results revealed that the binding affinities of BA to the core targets were lower than those of BE. The Log p-value of BE (1.7) was markedly higher than that of BA (-0.5). Furthermore, BE accumulated in cells at a level approximately 200 times greater than BA.
Conclusion: BE exhibits stronger anti-inflammatory activity relative to BA, possibly attributed to its better lipid solubility and cellular penetration capabilities. The anti-inflammatory mechanism of BE may be mediated through the PI3K-AKT pathway.
Keywords: baicalin butyl ester; inflammation; molecular docking; network pharmacology; traditional Chinese medicine.
Copyright © 2024 Du, Li, Su, He, Tan, Hou, He, Pan, Xu, Cao, Dong and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bao M. (2022). Comparative study of baicalin and baicalin n-butyl esterin alleviating LPS induced intestinal inflammation andoxidative stress in IPEC-J2 cells and mice[D]. China: Guangdong Ocean University. 10.27788/d.cnki.ggdhy.2022.000269 - DOI
-
- Brooke D., Nielsen I., de Bruijn J., Hermens J. (1990). An interlaboratory evaluation of the stir-flask method for the determination of octanol-water partition coefficients (Log Pow). Chemosphere 21 (1-2), 119–133. 10.1016/0045-6535(90)90385-7 - DOI
-
- Ebada H. M. K., Nasra M. M. A., Nassra R. A., Abdallah O. Y. (2022). Chondroitin sulfate-functionalized lipid nanoreservoirs: a novel cartilage-targeting approach for intra-articular delivery of cassic acid for osteoarthritis treatment. Drug Deliv. 29 (1), 652–663. 10.1080/10717544.2022.2041130 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

