Affimer reagents enable targeted delivery of therapeutic agents and RNA via virus-like particles
- PMID: 39104409
- PMCID: PMC11298639
- DOI: 10.1016/j.isci.2024.110461
Affimer reagents enable targeted delivery of therapeutic agents and RNA via virus-like particles
Abstract
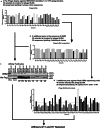
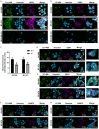
Monoclonal antibodies have revolutionized therapies, but non-immunoglobulin scaffolds are becoming compelling alternatives owing to their adaptability. Their ability to be labeled with imaging or cytotoxic compounds and to create multimeric proteins is an attractive strategy for therapeutics. Focusing on HER2, a frequently overexpressed receptor in breast cancer, this study addresses some limitations of conventional targeting moieties by harnessing the potential of these scaffolds. HER2-binding Affimers were isolated and characterized, demonstrating potency as binding reagents and efficient internalization by HER2-overexpressing cells. Affimers conjugated with cytotoxic agent achieved dose-dependent reductions in cell viability within HER2-overexpressing cell lines. Bispecific Affimers, targeting HER2 and virus-like particles, facilitated efficient internalization of virus-like particles carrying enhanced green fluorescent protein (eGFP)-encoding RNA, leading to protein expression. Anti-HER2 affibody or designed ankyrin repeat protein (DARPin) fusion constructs with the anti-VLP Affimer further underscore the adaptability of this approach. This study demonstrates the versatility of scaffolds for precise delivery of cargos into cells, advancing biotechnology and therapeutic research.
Keywords: Molecular biology; Virology.
© 2024 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare a potential conflict of interest. A patent application has been filed related to the work described in this manuscript. [Application number: LU505126, Inventors: George P. Lomonossoff, Sachin Shah, Keith Saunders, Darren C Tomlinson, Christian Tiede, Heather Martin, Ross Overman]. D.C.T. also is a named inventor on patent “scaffold protein derived from plant cystatins” Europe 14705565.1, US 14/768,032, Australia 2014217628. G.P.L. also sits as a chair on the advisory board for Leaf Expression Systems.
Figures




References
-
- IARC . World Health Organisation; 2020. World Cancer Report 2020.
-
- Gianni L., Pienkowski T., Im Y.H., Roman L., Tseng L.M., Liu M.C., Lluch A., Staroslawska E., de la Haba-Rodriguez J., Im S.A., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/s1470-2045(11)70336-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

