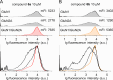
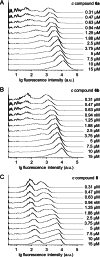
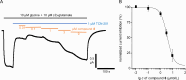
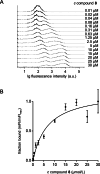
A novel fluorescent labeling compound for GluN2A containing N-methyl-d-aspartate receptors identified by autodisplay-based screening
- PMID: 39104865
- PMCID: PMC11298907
- DOI: 10.1016/j.jpha.2024.01.013
A novel fluorescent labeling compound for GluN2A containing N-methyl-d-aspartate receptors identified by autodisplay-based screening
Abstract
Image 1.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



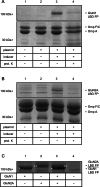
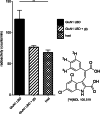
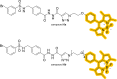
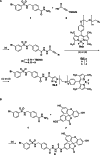

References
-
- Furukawa H., Singh S.K., Mancusso R., et al. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. - PubMed
LinkOut - more resources
Full Text Sources

