Screening of Diagnostic Biomarkers and Immune Infiltration Characteristics Linking Rheumatoid Arthritis and Rosacea Based on Bioinformatics Analysis
- PMID: 39104909
- PMCID: PMC11299729
- DOI: 10.2147/JIR.S467760
Screening of Diagnostic Biomarkers and Immune Infiltration Characteristics Linking Rheumatoid Arthritis and Rosacea Based on Bioinformatics Analysis
Abstract
Introduction: Both rheumatoid arthritis (RA) and rosacea represent common chronic systemic autoimmune conditions. Recent research indicates a heightened RA risk among individuals with rosacea. However, the molecular mechanisms linking these diseases remain largely unknown. This study aims to uncover shared molecular regulatory networks and immune cell infiltration patterns in both rosacea and RA.
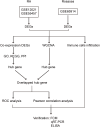
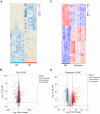
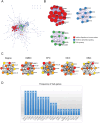
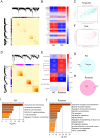
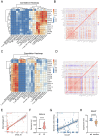
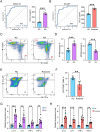
Methods: The gene expression profiles of RA (GSE12021, GSE55457), and the rosacea gene expression profile (GSE6591), were downloaded from Gene Expression Omnibus (GEO) databases, and obtained to screen differentially expressed genes (DEGs) by using "limma" package in R software. Various analyses including GO, KEGG, protein-protein interaction (PPI) network, and weighted gene co-expression network analyses (WGCNA) were conducted to explore potential biological functions and signaling pathways. CIBERSORT was used to assess the abundance of immune cells. Pearson coefficients were used to calculate the correlations between overlapped genes and the leukocyte gene signature matrix. Flow cytometry (FCM) analysis confirmed the most abundant immune cells detected in rheumatoid arthritis and rosacea. Receiver operator characteristic (ROC) analysis, enzyme-linked immunosorbent assay (ELISA), and qRT-PCR were used to confirm biomarkers and functions.
Results: Two hundred seventy-seven co-expressed DEGs were identified from these datasets. Functional enrichment analysis indicated that these DEGs were associated with immune processes and chemokine-mediated signaling pathways. Fourteen and 17 hub genes overlapped between cytoHubba and WGCNA were identified in RA and rosacea, respectively. Macrophages and dendritic cells were RA and rosacea's most abundant immune cells, respectively. The ROC curves demonstrated potential diagnostic values of CXCL10 and CCL27, showing higher levels in the serum of patients with RA or rosacea, and suggesting possible regulation in the densities and functions of macrophages and dendritic cells from RA and rosacea, which were validated by FCM and qRT-PCR.
Conclusion: Importantly, our findings may contribute to the scientific basis for biomarkers and therapeutic targets for patients with RA and rosacea in the future.
Keywords: autoimmune disease; chemokine; diagnostic biomarkers; immune infiltration; macrophage.
© 2024 Wang et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures


References
-
- Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: A Synopsis. Am J Manag Care. 2014;20(7):S128–135. - PubMed
LinkOut - more resources
Full Text Sources

