Ravulizumab in Atypical Hemolytic Uremic Syndrome: An Analysis of 2-Year Efficacy and Safety Outcomes in 2 Phase 3 Trials
- PMID: 39105067
- PMCID: PMC11298908
- DOI: 10.1016/j.xkme.2024.100855
Ravulizumab in Atypical Hemolytic Uremic Syndrome: An Analysis of 2-Year Efficacy and Safety Outcomes in 2 Phase 3 Trials
Abstract
Rationale & objective: Atypical hemolytic uremic syndrome (aHUS) is a rare form of thrombotic microangiopathy (TMA) caused by complement dysregulation. Ravulizumab is a C5i approved for the treatment of aHUS. This analysis assessed long-term outcomes of ravulizumab in adults and pediatric patients with aHUS.
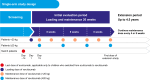
Study design: This analysis reports 2-year data from 2 phase 3, single-arm studies.
Setting & participants: One study included C5i-naïve adults (NCT02949128), and the other included 2 cohorts of pediatric patients (C5i-naïve and those who switched to ravulizumab from eculizumab [pediatric switch patients]; NCT03131219).
Exposure: Patients received intravenous ravulizumab every 4-8 weeks, with the dose depending on body weight.
Outcomes: The primary endpoint in the studies of C5i-naïve patients was complete TMA response, which consisted of platelet count normalization, lactate dehydrogenase normalization, and ≥25% improvement in serum creatinine concentrations from baseline, at 2 consecutive assessments ≥4 weeks apart.
Analytical approach: All analyses used descriptive statistics. No formal statistical comparisons were performed.
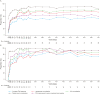
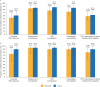
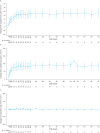
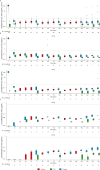
Results: In total, 86 and 92 patients were included in efficacy and safety analyses, respectively. Complete TMA response rates over 2 years were 61% and 90% in C5i-naïve adults and pediatric patients, respectively. The median increase in estimated glomerular filtration rate from baseline was maintained over 2 years in C5i-naïve adults (35 mL/min/1.73 m2) and pediatric patients (82.5 mL/min/1.73 m2). Most adverse events and serious adverse events occurred during the first 26 weeks. No meningococcal infections were reported. Improvement in the Functional Assessment of Chronic Illness Therapy - Fatigue score achieved by 26 weeks was maintained over 2 years.
Limitations: Limitations were the small sample of pediatric switch patients and limited availability of genetic data.
Conclusions: Long-term treatment with ravulizumab is well tolerated and associated with improved hematologic and renal parameters and quality of life in adults and pediatric patients with aHUS.
Keywords: Adult; atypical hemolytic uremic syndrome; complement C5 inhibitor; efficacy; hematology; nephrology; pediatric; ravulizumab; safety; thrombotic microangiopathy.
Plain language summary
This research tested a drug called ravulizumab for the treatment of atypical hemolytic uremic syndrome (aHUS). aHUS is a rare disease that causes clots in tiny blood vessels. This can damage the kidneys and other organs. We analyzed data from 2 clinical trials in which children and adults with aHUS received ravulizumab through a tube placed in a vein (intravenous line). They received ravulizumab every 4-8 weeks depending on their weight. We found that treating patients for 2 years with ravulizumab was associated with improved blood health, kidney function, and quality of life and was well tolerated. These results support ravulizumab as a long-term treatment for people with aHUS.
© 2024 The Authors.
Figures

References
-
- Goodship T.H., Cook H.T., Fakhouri F., et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91(3):539–551. - PubMed
-
- Fakhouri F., Zuber J., Frémeaux-Bacchi V., Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681–696. - PubMed
-
- Raina R., Krishnappa V., Blaha T., et al. Atypical hemolytic-uremic syndrome: an update on pathophysiology, diagnosis, and treatment. Ther Apher Dial. 2019;23(1):4–21. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

