Caspases compromise SLU7 and UPF1 stability and NMD activity during hepatocarcinogenesis
- PMID: 39105183
- PMCID: PMC11298840
- DOI: 10.1016/j.jhepr.2024.101118
Caspases compromise SLU7 and UPF1 stability and NMD activity during hepatocarcinogenesis
Abstract
Background & aims: The homeostasis of the cellular transcriptome depends on transcription and splicing mechanisms. Moreover, the fidelity of gene expression, essential to preserve cellular identity and function is secured by different quality control mechanisms including nonsense-mediated RNA decay (NMD). In this context, alternative splicing is coupled to NMD, and several alterations in these mechanisms leading to the accumulation of aberrant gene isoforms are known to be involved in human disease including cancer.
Methods: RNA sequencing, western blotting, qPCR and co-immunoprecipitation were performed in multiple silenced culture cell lines (replicates n ≥4), primary hepatocytes and samples of animal models (Jo2, APAP, Mdr2 -/- mice, n ≥3).
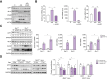
Results: Here we show that in animal models of liver injury and in human HCC (TCGA, non-tumoral = 50 vs. HCC = 374), the process of NMD is inhibited. Moreover, we demonstrate that the splicing factor SLU7 interacts with and preserves the levels of the NMD effector UPF1, and that SLU7 is required for correct NMD. Our previous findings demonstrated that SLU7 expression is reduced in the diseased liver, contributing to hepatocellular dedifferentiation and genome instability during disease progression. Here we build on this by providing evidence that caspases activated during liver damage are responsible for the cleavage and degradation of SLU7.
Conclusions: Here we identify the downregulation of UPF1 and the inhibition of NMD as a new molecular pathway contributing to the malignant reshaping of the liver transcriptome. Moreover, and importantly, we uncover caspase activation as the mechanism responsible for the downregulation of SLU7 expression during liver disease progression, which is a new link between apoptosis and hepatocarcinogenesis.
Impact and implications: The mechanisms involved in reshaping the hepatocellular transcriptome and thereby driving the progressive loss of cell identity and function in liver disease are not completely understood. In this context, we provide evidence on the impairment of a key mRNA surveillance mechanism known as nonsense-mediated mRNA decay (NMD). Mechanistically, we uncover a novel role for the splicing factor SLU7 in the regulation of NMD, including its ability to interact and preserve the levels of the key NMD factor UPF1. Moreover, we demonstrate that the activation of caspases during liver damage mediates SLU7 and UPF1 protein degradation and NMD inhibition. Our findings identify potential new markers of liver disease progression, and SLU7 as a novel therapeutic target to prevent the functional decay of the chronically injured organ.
Keywords: apoptosis; hepatocellular carcinoma; liver damage; splicing; transcriptome.
© 2024 The Authors.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

