A red blood cell-based score in the prognostication of patients with metastatic RCC of the Meet-URO 15 study
- PMID: 39105621
- PMCID: PMC11486140
- DOI: 10.1080/1750743X.2024.2382666
A red blood cell-based score in the prognostication of patients with metastatic RCC of the Meet-URO 15 study
Abstract
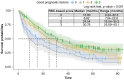
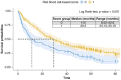
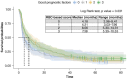
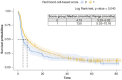
Aims: Anemia, mean corpuscular volume and red cell distribution width may have some effects on survival outcomes of metastatic renal cell carcinoma (mRCC) patients and are incorporated in a red blood cell (RBC)-based score. Its validity in prognostication of mRCC patients treated with second-line nivolumab was assessed.Patients and methods: Retrospective analysis using Meet-URO-15 cohort of mRCC patients receiving nivolumab in the second-line setting or beyond. Outcomes were overall survival (OS) and progression-free survival (PFS).Results: A total of 390 patients were included. Significant differences in OS and PFS between RBC-based score groups, with group 1 (2 or 3 of the RBC-related prognostic factors) having longer OS (median 29.5 months, 95% CI: 23.1-35.9, versus 11.5 months, 95% CI: 8.5-22.6; p < 0.001) and PFS (7.5 months, 95% CI: 5.5-10.2, versus 4.2 months, 95% CI: 3.3-5.9; p = 0.040) than those in group 0 (0 or 1 RBC-related prognostic factors). Belonging to group 1 independently predicted OS (hazard ratio: 0.65, 95% CI: 0.50-0.85; p = 0.002) but not PFS (hazard ratio: 0.89, 95% CI: 0.70-1.14, p = 0.370) or disease response (OR 0.68, 95% CI: 0.41-1.10; p = 0.118) at multivariable analysis.Conclusion: RBC-based group scores independently predicted OS in mRCC patients treated with nivolumab.
Keywords: metastatic renal cell carcinoma; overall survival; prognosis; progression-free survival; red blood cell-based score.
Plain language summary
This study looked at how certain blood cell measurements (anemia, mean corpuscular volume, red cell distribution width) affect survival in patients with metastatic renal cell carcinoma who are treated with nivolumab, an immunotherapy drug. Researchers analyzed data from 390 patients who received nivolumab as a second-line treatment or beyond. They divided patients into groups based on their blood cell scores. They found that patients with higher scores had better overall survival and progression-free survival compared with those with lower scores. Specifically, patients with better scores lived longer. The study concluded that these scores can help predict survival in these patients treated with nivolumab.
Conflict of interest statement
S Anpalakhan received support for conference travel and accommodation fees from Astellas. GL Banna reports personal fees outside the submitted work for advisory role, speaker engagements from Astellas, Astrazeneca, Amgen, Bayer and for travel and accommodation expenses from Merck, Janssen. S Buti received honoraria as a speaker at scientific events and advisory role by Bristol-Myers Squibb (BMS), Pfizer, MSD, Merck, Gentili, Ipsen and Novartis; he also received research funding from Novartis and Pfizer, but he can confirm that these grants do not interfere at all with this manuscript and the presented data. G Fornarini services advisory boards for Astellas, Janssen, Pfizer, Bayer, MSD, Merck and received travel accommodation from Astellas, Janssen, Bayer. SE Rebuzzi received honoraria as a speaker at scientific events and travel accommodation from BMS, Amgen, GSK, Ipsen, Astellas, Janssen, MSD. P Rescigno services advisory boards for MSD, AstraZeneca and Janssen. PA Zucali reports personal fees outside the submitted work for advisory role, speaker engagements and travel and accommodation expenses from Merck Sharp & Dohme (MSD), Astellas, Janssen, Sanofi, Ipsen, Pfizer, Novartis, Bristol Meyer Squibb, Amgen, Astra Zeneca, Roche and Bayer. All the other authors report no conflict of interests. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
