Marginated Neutrophils in the Lungs Effectively Compete for Nanoparticles Targeted to the Endothelium, Serving as a Part of the Reticuloendothelial System
- PMID: 39105696
- PMCID: PMC11935960
- DOI: 10.1021/acsnano.4c06286
Marginated Neutrophils in the Lungs Effectively Compete for Nanoparticles Targeted to the Endothelium, Serving as a Part of the Reticuloendothelial System
Abstract
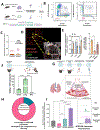
Nanomedicine has long pursued the goal of targeted delivery to specific organs and cell types but has yet to achieve this goal with the vast majority of targets. One rare example of success in this pursuit has been the 25+ years of studies targeting the lung endothelium using nanoparticles conjugated to antibodies against endothelial surface molecules. However, here we show that such "endothelial-targeted" nanocarriers also effectively target the lungs' numerous marginated neutrophils, which reside in the pulmonary capillaries and patrol for pathogens. We show that marginated neutrophils' uptake of many of these "endothelial-targeted" nanocarriers is on par with endothelial uptake. This generalizes across diverse nanomaterials and targeting moieties and was even found with physicochemical lung tropism (i.e., without targeting moieties). Further, we observed this in ex vivo human lungs and in vivo healthy mice, with an increase in marginated neutrophil uptake of nanoparticles caused by local or distant inflammation. These findings have implications for nanomedicine development for lung diseases. These data also suggest that marginated neutrophils, especially in the lungs, should be considered a major part of the reticuloendothelial system (RES), with a special role in clearing nanoparticles that adhere to the lumenal surfaces of blood vessels.
Keywords: lipid nanoparticles; lung targeting; lungs; marginated neutrophils; neutrophils; reticuloendothelial system.
Figures






References
-
- Wilhelm S; Tavares AJ; Dai Q; Ohta S; Audet J; Dvorak HF; Chan WCW Analysis of Nanoparticle Delivery to Tumours. Nature Reviews Materials 2016, 1 (5), 1–12.
-
- Ouyang B; Poon W; Zhang Y-N; Lin ZP; Kingston BR; Tavares AJ; Zhang Y; Chen J; Valic MS; Syed AM; MacMillan P; Couture-Senécal J; Zheng G; Chan WCW The Dose Threshold for Nanoparticle Tumour Delivery. Nat. Mater. 2020, 19 (12), 1362–1371. - PubMed
-
- Bachmaier K; Stuart A; Singh A; Mukhopadhyay A; Chakraborty S; Hong Z; Wang L; Tsukasaki Y; Maienschein-Cline M; Ganesh BB; Kanteti P; Rehman J; Malik AB Albumin Nanoparticle Endocytosing Subset of Neutrophils for Precision Therapeutic Targeting of Inflammatory Tissue Injury. ACS Nano 2022, 16 (3), 4084–4101. - PMC - PubMed
-
- Chen S-J; Sanmiguel J; Lock M; McMenamin D; Draper C; Limberis MP; Kassim SH; Somanathan S; Bell P; Johnston JC; Rader DJ; Wilson JM Biodistribution of AAV8 Vectors Expressing Human Low-Density Lipoprotein Receptor in a Mouse Model of Homozygous Familial Hypercholesterolemia. Hum Gene Ther Clin Dev 2013, 24 (4), 154–160. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

