GP100 expression is variable in intensity in melanoma
- PMID: 39105816
- PMCID: PMC11303354
- DOI: 10.1007/s00262-024-03776-5
GP100 expression is variable in intensity in melanoma
Abstract
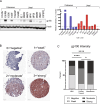
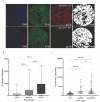
Drugs or cellular products that bind to gp100 are being investigated for treatment of cutaneous melanoma. The relative specificity of gp100 expression in melanocytes makes it an attractive target to harness for therapeutic intent. For example, Tebentafusp, a bispecific gp100 peptide-HLA-directed CD3 T cell engager, has generated significant enthusiasm in recent years due to its success in improving outcomes for uveal melanoma and is being studied in cutaneous melanoma. However, the extent and intensity of gp100 expression in advanced cutaneous melanoma has not been well studied. Here, we interrogated a large cohort of primary and metastatic melanomas for gp100 expression by immunohistochemistry. Expression in metastatic samples was globally higher and almost uniformly positive, however the degree of intensity was variable. Using a quantitative immunofluorescence method, we confirmed the variability in expression. As gp100-binding drugs are assessed in clinical trials, the association between activity of the drugs and the level of gp100 expression should be studied in order to potentially improve patient selection.
Keywords: Glycoprotein 100; ImmTAC; Melanoma; Targeted therapy; Tebentafusp.
© 2024. The Author(s).
Conflict of interest statement
H.M.K. holds Consulting or Advisory Roles with Iovance Biotherapeutics, Merck, Clinigen Group, Bristol Myers Squibb, ChemoCentryx, Signatera, Gigagen, GI Reviewers, Seranova Bio, Pliant, Eisai, Invox, Werewolf Pharma, has received research funding from Merck (Inst), Bristol Myers Squibb (Inst), Apexigen (Inst), and has received travel, accomodations, or expenses from Apexigen. No other potential competing interests were reported.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

