Unlocking the potential of HHLA2: identifying functional immune infiltrating cells in the tumor microenvironment and predicting clinical outcomes in laryngeal squamous cell carcinoma
- PMID: 39105870
- PMCID: PMC11303638
- DOI: 10.1007/s00262-024-03791-6
Unlocking the potential of HHLA2: identifying functional immune infiltrating cells in the tumor microenvironment and predicting clinical outcomes in laryngeal squamous cell carcinoma
Abstract
Background: HHLA2 (human endogenous retrovirus-H long terminal repeat-associating protein 2) represents a recently identified member of the B7 immune checkpoint family, characterized by limited expression in normal tissues but notable overexpression in various cancer types. Nevertheless, the precise function and interaction with immune cells remain poorly understood, particularly in laryngeal squamous cell carcinoma (LSCC). This investigation endeavored to elucidate the biological significance of HHLA2 within the tumor microenvironment of human LSCC tissues and delineate the clinical relevance and functional roles of HHLA2 in LSCC pathogenesis.
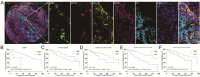
Methods: Through multiplexed immunohistochemistry analyses conducted on tissue microarrays sourced from LSCC patients (n = 72), the analysis was executed to assess the expression levels of HHLA2, density and spatial patterns of CD68+HLA-DR+CD163- (M1 macrophages), CTLA-4+CD4+FoxP3+ (CTLA-4+Treg cells), CTLA-4+CD4+FoxP3- (CTLA-4+Tcon cells), exhausted CD8+T cells, and terminally exhausted CD8+T cells in LSCC tissues. Survival analysis was conducted to evaluate the prognostic significance of HHLA2 and these immune checkpoints or immune cell populations, employing COX regression analysis to identify independent prognostic factors.
Results: Kaplan-Meier (K-M) survival curves revealed a significant association between HHLA2 expression and overall survival (OS) in LSCC. Elevated levels of HHLA2 were linked to reduced patient survival, indicating its potential as a prognostic marker (HR: 3.230, 95%CI 0.9205-11.34, P = 0.0067). Notably, increased infiltration of CD68+ cells (total macrophages), STING+CD68+HLA-DR+CD163- (STING+M1 macrophages), CTLA-4+CD4+FoxP3+, CTLA-4+CD4+FoxP3-, PD-1+LAG-3+CD8+T cells, and PD-1+LAG-3+TIM-3+CD8+T cells strongly linked to poorer survival outcomes (P < 0.05). A discernible trend was observed between the levels of these immune cell populations, STING+CD68+ (STING+ total macrophages), CD68+HLA-DR+CD163-, STING+CD68+CD163+HLA-DR- (STING+M2 macrophages), PD-1+LAG-3-CD8+T cells, PD-1+TIM-3+CD8+T cells, and PD-1+LAG-3+TIM-3-CD8+T cells and prognosis. Importantly, multivariate COX analysis identified HHLA2 as an independent predictive factor for OS in LSCC patients (HR = 3.86, 95% CI 1.08-13.80, P = 0.038). This underscored the potential of HHLA2 as a critical marker for predicting patient outcomes in LSCC.
Conclusions: HHLA2 emerged as a detrimental prognostic biomarker for assessing OS in LSCC patients. Relative to other immune checkpoints, HHLA2 exhibited heightened predictive efficacy for the prognosis of LSCC patients.
Keywords: HHLA2; Immune infiltrating cell; Laryngeal squamous cell carcinoma; Prognosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Horozoglu C, Sonmez D, Demirkol S et al (2021) Potential role of immune cell genetic variants associated with tumor microenvironment response in laryngeal squamous cell carcinoma (LSCC) in terms of clinicopathological features. Pathol Res Pract 228:153665. 10.1016/j.prp.2021.153665 10.1016/j.prp.2021.153665 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

