Safety and clinical activity of JNJ-78306358, a human leukocyte antigen-G (HLA-G) x CD3 bispecific antibody, for the treatment of advanced stage solid tumors
- PMID: 39105878
- PMCID: PMC11303617
- DOI: 10.1007/s00262-024-03790-7
Safety and clinical activity of JNJ-78306358, a human leukocyte antigen-G (HLA-G) x CD3 bispecific antibody, for the treatment of advanced stage solid tumors
Abstract
Background: JNJ-78306358 is a bispecific antibody that redirects T cells to kill human leukocyte antigen-G (HLA-G)-expressing tumor cells. This dose escalation study evaluated the safety, pharmacokinetics, pharmacodynamics, and preliminary antitumor activity of JNJ-78306358 in patients with advanced solid tumors.
Methods: Adult patients with metastatic/unresectable solid tumors with high prevalence of HLA-G expression were enrolled. Dose escalation was initiated with once-weekly subcutaneous administration with step-up dosing to mitigate cytokine release syndrome (CRS).
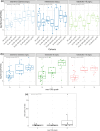
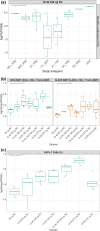
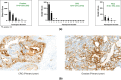
Results: Overall, 39 heavily pretreated patients (colorectal cancer: n = 23, ovarian cancer: n = 10, and renal cell carcinoma: n = 6) were dosed in 7 cohorts. Most patients (94.9%) experienced ≥ 1 treatment-emergent adverse events (TEAEs); 87.2% had ≥ 1 related TEAEs. About half of the patients (48.7%) experienced CRS, which were grade 1/2. Nine patients (23.1%) received tocilizumab for CRS. No grade 3 CRS was observed. Dose-limiting toxicities (DLTs) of increased transaminases, pneumonitis and recurrent CRS requiring a dose reduction were reported in 4 patients, coinciding with CRS. No treatment-related deaths reported. No objective responses were noted, but 2 patients had stable disease > 40 weeks. JNJ-78306358 stimulated peripheral T cell activation and cytokine release. Anti-drug antibodies were observed in 45% of evaluable patients with impact on exposure. Approximately half of archival tumor samples (48%) had expression of HLA-G by immunohistochemistry.
Conclusion: JNJ-78306358 showed pharmacodynamic effects with induction of cytokines and T cell activation. JNJ-78306358 was associated with CRS-related toxicities including increased transaminases and pneumonitis which limited its dose escalation to potentially efficacious levels. Trial registration number ClinicalTrials.gov (No. NCT04991740).
Keywords: Cytokine release syndrome; Dose escalation study; Human leukocyte antigen-G; JNJ-78306358; Phase 1; Solid tumors.
© 2024. The Author(s).
Conflict of interest statement
Revit Geva: Consultant for AstraZeneca, Roche, Bayer, MSD, Oncotest, and Pfizer; Leadership role with Pyxis; Shareholder/stockholder/stock options with Pyxis; Honoraria from AstraZeneca, Amgen, Janssen, Medison, Merck, MSD, Novartis, Pfizer, Takeda, and Roche; Travel/accommodation expenses from Medison and Merck. Maria Vieito: Consultant for Debiopharm Group, Roche, and TFS; Travel/ accommodation expenses from Merck, Roche, Serono. Ruth Perets: Consultant for Clexio Biosciences, Galmed Pharmaceuticals, IE therapeutics, and Simplivia; Honoraria from MSD and GSK; Travel/accommodation expenses from MSD. Emiliano Calvo: Employee of START; Leadership role with BeiGene, EORTC, Merus NV, Novartis, PharmaMar, Sanofi, and START; Shareholder/stockholder/stock options with Oncoart Associated and START; Honoraria from HM Hospitales Group; Consultant for Adcendo, Amunix, Anaveon, AstraZeneca/MedImmune, Bristol-Myers Squibb, Chugai Pharma, Diaccurate, Elevation Oncology, Ellipses Pharma, Genmab, Janssen-Cilag, MonTa, MSD Oncology, Nanobiotix, Nouscom, Novartis, OncoDNA, PharmaMar, Roche/Genentech, Servier, Syneos Health, T-Knife, and TargImmune Therapeutics; Research funding from START; President and Founder of Foundation INTHEOS (Investigational Therapeutics in Oncological Sciences); Member of Non-for-profit Foundation PharmaMar, Non-for-profit CRIS Cancer Foundation. Elena Garralda: Employee of NEXT Oncology; consultant or advisor to Alkermes, Anaveon, Boehringer Ingelheim, Ellipses Pharma, F-Star Therapeutics, Hengrui Therapeutics, Incyte, Janssen, MabDiscovery, Roche, Sanofi, Seattle Genetics, and Thermo Fisher Scientific; Speakers bureau of Eli Lilly, MSD, Novartis, Roche, Seagen, and Thermo Fisher Scientific; Recipient of numerous institutional research grants. Victor Moreno: Employee of START; Consultant for Bayer, BMS, Janssen Oncology, and Merck; Speaker bureau fees from Bayer; Research funding from Abbvie, ACEA Biosciences, Adaptimmune, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, BMS, Celgene, E-therapeutics, Eisai, GSK, Janssen, Menarini, Merck, Nanobiotix, Novartis, Pfizer, PharmaMar, PsiOxus Therapeutics, Puma Biotechnology, Regeneron, RigonTEC, Roche, Sanofi, Sierra Oncology, Synthon, Taiho Pharmaceutical, Takeda, Tesaro, and Transgene; Travel and accommodation expenses from Regeneron/Sanofi. Regina J. Brown, James G. Greger, Shujian Wu, Douglas Steinbach, Tsun-Wen Sheena Yao, Yu Cao, Josh Lauring, Ruchi Chaudhary, Jaymala Patel and Bharvin Patel: Employees of Janssen Research & Development and own stock/stock options in Johnson & Johnson. Jorge Ramon, Manuel Pedregal, Elena Corral, Bernard Doger and Jorge Bardina: No conflict of interest.
Figures



References
-
- US Food and Drug Administration (2023) Bispecific antibodies: an area of research and clinical applications. Retrieved from: https://www.fda.gov/drugs/news-events-human-drugs/bispecific-antibodies-.... Accessed Nov 9, 2023
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

