A Phase Ib/II Randomized Clinical Trial of Oleclumab with or without Durvalumab plus Chemotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma
- PMID: 39106081
- PMCID: PMC11474165
- DOI: 10.1158/1078-0432.CCR-24-0499
A Phase Ib/II Randomized Clinical Trial of Oleclumab with or without Durvalumab plus Chemotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma
Abstract
Purpose: Pancreatic ductal adenocarcinoma upregulates CD73, potentially contributing to immune surveillance evasion. Combining oleclumab (CD73 inhibitor) and durvalumab with chemotherapy may identify an effective treatment option.
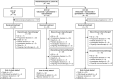
Patients and methods: We describe a multicenter phase Ib/II randomized clinical trial in patients with metastatic pancreatic ductal adenocarcinoma, untreated (cohort A) or previously received gemcitabine-based chemotherapy (cohort B; NCT03611556). During escalation, patients received oleclumab 1,500 or 3,000 mg, durvalumab 1,500 mg, and gemcitabine plus nab-paclitaxel (GnP; cohort A; n = 14) or modified FOLFOX (cohort B; n = 11). During expansion, cohort A patients (n = 170) were randomized to GnP (arm A1), oleclumab [recommended phase II dose (RP2D)] with GnP (arm A2), or oleclumab (RP2D) with durvalumab plus GnP (arm A3). Primary objectives were safety (escalation) and objective response rate (expansion). Secondary objectives included progression-free survival (PFS) and overall survival (OS).
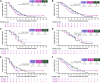
Results: During escalation, 1/11 patients from cohort B (oleclumab 3,000 mg) experienced two dose-limiting toxicities. Oleclumab's RP2D was 3,000 mg. During expansion, grade ≥3 treatment-related adverse events occurred in 67.7% (42/62) of patients in A1, 73.7% (28/38) in A2, and 77.1% (54/70) in A3. The objective response rate was 29.0%, 21.1%, and 32.9% in A1, A2, and A3, respectively (A1 vs. A3; P = 0.650). PFS [HR = 0.72; 95% confidence interval (CI), 0.47, 1.11] and OS (HR = 0.75; 95% CI, 0.50-1.13) were similar for A3 versus A1. Patients with high CD73 expression had improved PFS and OS in A3 versus A1, although this should be interpreted with caution.
Conclusions: Although the safety profile was acceptable, this study did not meet its primary efficacy endpoint.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A.L. Coveler reports grants and nonfinancial support from AstraZeneca during the conduct of the study, as well as grants from Amgen, Novocure, Surface Oncology, Nextrast, and Actuate and grants and nonfinancial support from Seagen/Pfizer, AbGenomics, and NuCana outside the submitted work. M.J. Reilley reports personal fees from Pfizer, Seattle Genetics, and NEMJ and grants from AstraZeneca, Cardiff Oncology, MacroGenics, Surface Oncology, Takeda, and Xencor outside the submitted work. M. Zalupski reports grants from MedImmune during the conduct of the study. T. Macarulla reports personal fees from Ability Pharmaceuticals SL, Arcus Biosciences Inc., AstraZeneca, Basilea Pharmaceutica, Baxter, BioLineRx Ltd., Celgene, Eisai, Incyte, Ipsen Bioscience Inc., Janssen, Eli Lilly and Company, Esteve, MSD, Novocure, QED Therapeutics, Roche Farma, Sanofi-Aventis, Servier, and Zymeworks and personal fees and other support from Daiichi, BioNTech, Novartis, and Jazz Pharmaceuticals outside the submitted work. C. Fountzilas reports grants from AstraZeneca during the conduct of the study; grants from NCI, Merck, National Comprehensive Cancer Network Foundation, and National Comprehensive Cancer Network Oncology Research Program and other support from Taiho Oncology and Pfizer Inc. outside the submitted work; and consultation services to AstraZeneca (paid to institute) outside of the scope of the current work, >36 months prior to publication. M. Ponz-Sarvisé reports grants, personal fees, and nonfinancial support from AstraZeneca, grants from Novocure, grants and nonfinancial support from Roche, and personal fees from Taiho outside the submitted work. A. Nagrial reports grants and personal fees from Bristol Myers Squibb and personal fees from MSD, AstraZeneca, BeiGene, Roche, Astellas, and Janssen outside the submitted work. N.V. Uboha reports personal fees from AstraZeneca, Astellas, Bristol Myers Squibb, Eli Lilly and Company, Elevation Oncology, Merck, and Eisai, grants and personal fees from Ipsen and Arcus, and grants from EMD Serono outside the submitted work. M. Overman reports personal fees from Roche, MedImmune, Takeda, Bristol Myers Squibb, Janssen, Pfizer, 3DMed, and Merck outside the submitted work. A. Noonan reports other support from MedImmune during the conduct of the study, as well as other support from AstraZeneca, DAVA Oncology, Taiho Oncology, Elevar Therapeutics, and Bexion outside the submitted work. W.A. Messersmith reports grants from MedImmune during the conduct of the study. N. Pavlakis reports personal fees from MSD, Merck, Bristol Myers Squibb, AstraZeneca, Takeda, Amgen, BeiGene, Gilead, and Zymeworks and grants and personal fees from Pfizer, Roche, and Bayer outside the submitted work. N.B. Mettu reports grants from Compass Therapeutics, Repare Therapeutics, BioMed Valley Discoveries, Amgen Inc., Sapience Therapeutics Inc., Merck Sharp and Dohme, Seagen Inc., NuCana plc, Actuate Therapeutics, NGM Biopharmaceuticals Inc., and Adanate outside the submitted work. I. Bisha reports personal fees from AstraZeneca during the conduct of the study, as well as personal fees from AstraZeneca outside the submitted work. E. Murtomaki reports full-time employment with AstraZeneca and holding stocks and options. A.A. Bielska reports personal fees from AstraZeneca during the conduct of the study and outside the submitted work. Z.A. Cooper reports personal fees and other support from AstraZeneca during the conduct of the study. R. Kumar reports a patent for anti-CD73 antibody pending, as well as ownership of AstraZeneca stock. D.R. Spigel reports grants from AstraZeneca during the conduct of the study, as well as grants and other support from AbbVie, Amgen, AstraZeneca, Daiichi Sankyo, Roche/Genentech, GlaxoSmithKline, Jazz Pharmaceuticals, Lyell Immunopharma, MedImmune, Novartis, and Novocure, grants from Bayer, BeiGene, BioNTech, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Cyteir Therapeutics, Agios, AnHeart Therapeutics, Apollomics, Arcus, Arrys Therapeutics, Ascendis Pharma, Asher Biotherapeutics, Astellas, Denovo Biopharma, Eisai, Elevation Oncology, Ellipses Pharma, EMD Serono, Endeavor, Erasca, Faeth Therapeutics, Foundation Bio, Fujifilm Pharmaceuticals, G1 Therapeutics, Gilead Sciences, GRAIL, Hutchison MediPharma, Incyte, Ipsen, Janssen, Janux Therapeutics, Kronos Bio, Eli Lilly and Company, Loxo Oncology, MacroGenics, Merck, Millennium Pharmaceuticals, Moderna, Molecular Templates, Monte Rosa Therapeutics, Nektar, Oncologie, Peloton Therapeutics, Phanes Therapeutics, PTC Therapeutics, PureTech Health, Razor Genomics, Repare Therapeutics, Rgenix, Seagen, Shenzhen Chipscreen Biosciences, Strata Oncology, Stemline Therapeutics, Synthekine, Taiho, Takeda Pharmaceuticals, Tango Therapeutics, Tarveda, Tizona Therapeutics, Verastem, and Zai Laboratory, and other support from Circle Pharma and ModeX Therapeutics outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12–49. - PubMed
-
- Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, et al. . PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett 2017;407:57–65. - PubMed
-
- Yamashita Y, Hooker SW, Jiang H, Laurent AB, Resta R, Khare K, et al. . CD73 expression and fyn-dependent signaling on murine lymphocytes. Eur J Immunol 1998;28:2981–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

