Hif-2α programs oxygen chemosensitivity in chromaffin cells
- PMID: 39106106
- PMCID: PMC11405041
- DOI: 10.1172/JCI174661
Hif-2α programs oxygen chemosensitivity in chromaffin cells
Abstract
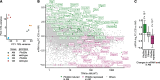
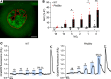
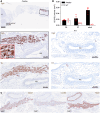
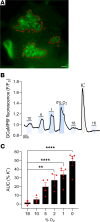
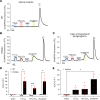
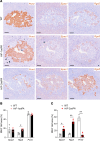
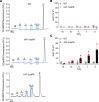
The study of transcription factors that determine specialized neuronal functions has provided invaluable insights into the physiology of the nervous system. Peripheral chemoreceptors are neurone-like electrophysiologically excitable cells that link the oxygen concentration of arterial blood to the neuronal control of breathing. In the adult, this oxygen chemosensitivity is exemplified by type I cells of the carotid body, and recent work has revealed one isoform of the hypoxia-inducible transcription factor (HIF), HIF-2α, as having a nonredundant role in the development and function of that organ. Here, we show that activation of HIF-2α, including isolated overexpression of HIF-2α but not HIF-1α, is sufficient to induce oxygen chemosensitivity in adult adrenal medulla. This phenotypic change in the adrenal medulla was associated with retention of extra-adrenal paraganglioma-like tissues resembling the fetal organ of Zuckerkandl, which also manifests oxygen chemosensitivity. Acquisition of chemosensitivity was associated with changes in the adrenal medullary expression of gene classes that are ordinarily characteristic of the carotid body, including G protein regulators and atypical subunits of mitochondrial cytochrome oxidase. Overall, the findings suggest that, at least in certain tissues, HIF-2α acts as a phenotypic driver for cells that display oxygen chemosensitivity, thus linking 2 major oxygen-sensing systems.
Keywords: Development; Embryonic development; Hypoxia; Oncology.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

