Validation of the Barcelona-MRI predictive model when PI-RADS v2.1 is used with trans-perineal prostate biopsies
- PMID: 39106115
- PMCID: PMC11446555
- DOI: 10.1590/S1677-5538.IBJU.2024.0204
Validation of the Barcelona-MRI predictive model when PI-RADS v2.1 is used with trans-perineal prostate biopsies
Abstract
Purpose: To validate the Barcelona magnetic resonance imaging predictive model (BCN-MRI PM) in men with pre-biopsy multiparametric MRI (mpMRI) reported with the Prostate Imaging Reporting and Data System (PI-RADS) v2.1, followed by transrectal and transperineal prostate biopsies.
Materials and methods: Prospective analysis of 3,264 men with PSA >3.0 ng/mL and/or abnormal digital rectal examination who were referred to ten participant centers in the csPCa early detection program of Catalonia (Spain), between 2021 and 2023. MpMRI was reported with the PI-RADS v2.1, and 2- to 4-core MRI-transrectal ultrasound (TRUS) fusion-targeted biopsy of suspected lesions and/or 12-core systematic biopsy were conducted. 2,295 (70.3%) individuals were referred to six centers for transrectal prostate biopsies, while 969 (39.7%) were referred to four centers for transperineal prostate biopsies. CsPCa was classified whenever the International Society of Urologic Pathology grade group was 2 or higher.
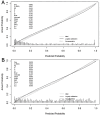
Results: CsPCa was detected in 41% of transrectal prostate biopsies and in 45.9% of transperineal prostate biopsies (p < 0.016). Both BCN-MRI PM calibration curves were within the ideal correlation between predicted and observed csPCa. Areas under the curve and 95% confidence intervals were 0.847 (0.830-0.857) and 0.830 (0.823-0.855), respectively (p = 0.346). Specificities corresponding to 95% sensitivity were 37.6 and 36.8%, respectively (p = 0.387). The Net benefit of the BCN-MRI PM was similar with both biopsy methods.
Conclusions: The BCN-MRI PM has been successfully validated when mpMRI was reported with the PI-RADS v2.1 and prostate biopsies were conducted via the transrectal and transperineal route.
Keywords: Diagnosis; Magnetic Resonance Imaging; Prostatic Neoplasms.
Copyright® by the International Brazilian Journal of Urology.
Conflict of interest statement
None declared.
Figures


References
-
- Van Poppel H, Roobol MJ, Chapple CR, Catto JWF, N'Dow J, Sønksen J, et al. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur Urol. 2021;80:703–711. doi: 10.1016/j.eururo.2021.07.024. - DOI - PubMed
-
- Van Poppel H, Roobol MJ, Chapple CR, Catto JWF, N'Dow J, Sønksen J, et al. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur Urol. 2021;80:703–711. doi: 10.1016/j.eururo.2021.07.024. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
