Versatile Method for Preparing Two-Dimensional Metal Dihalides
- PMID: 39106126
- PMCID: PMC11342368
- DOI: 10.1021/acsnano.4c04397
Versatile Method for Preparing Two-Dimensional Metal Dihalides
Abstract
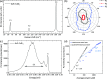
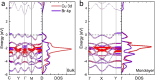
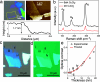
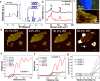
Ever since the ground-breaking isolation of graphene, numerous two-dimensional (2D) materials have emerged with 2D metal dihalides gaining significant attention due to their intriguing electrical and magnetic properties. In this study, we introduce an innovative approach via anhydrous solvent-induced recrystallization of bulk powders to obtain crystals of metal dihalides (MX2, with M = Cu, Ni, Co and X = Br, Cl, I), which can be exfoliated to 2D flakes. We demonstrate the effectiveness of our method using CuBr2 as an example, which forms large layered crystals. We investigate the structural properties of both the bulk and 2D CuBr2 using X-ray diffraction, along with Raman scattering and optical spectroscopy, revealing its quasi-1D chain structure, which translates to distinct emission and scattering characteristics. Furthermore, microultraviolet photoemission spectroscopy and electronic transport reveal the electronic properties of CuBr2 flakes, including their valence band structure. We extend our methodology to other metal halides and assess the stability of the metal halide flakes in controlled environments. We show that optical contrast can be used to characterize the flake thicknesses for these materials. Our findings demonstrate the versatility and potential applications of the proposed methodology for preparing and studying 2D metal halide flakes.
Keywords: Raman scattering spectroscopy; mechanical exfoliation; metal dihalides; photoemission; solvent-assisted recrystallization; two-dimensional materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Anasori B.; Lukatskaya M. R.; Gogotsi Y. 2d Metal Carbides and Nitrides (Mxenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098.10.1038/natrevmats.2016.98. - DOI
-
- Manzeli S.; Ovchinnikov D.; Pasquier D.; Yazyev O. V.; Kis A. 2d Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033.10.1038/natrevmats.2017.33. - DOI
-
- Mounet N.; Gibertini M.; Schwaller P.; Campi D.; Merkys A.; Marrazzo A.; Sohier T.; Castelli I. E.; Cepellotti A.; Pizzi G.; et al. Two-Dimensional Materials from High-Throughput Computational Exfoliation of Experimentally Known Compounds. Nat. Nanotechnol. 2018, 13, 246–252. 10.1038/s41565-017-0035-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources

