Efficacy and safety of probiotics in IBD: An overview of systematic reviews and updated meta-analysis of randomized controlled trials
- PMID: 39106167
- PMCID: PMC11497663
- DOI: 10.1002/ueg2.12636
Efficacy and safety of probiotics in IBD: An overview of systematic reviews and updated meta-analysis of randomized controlled trials
Abstract
Background and objective: Probiotics show promise in inflammatory bowel disease (IBD), yet knowledge gaps persist. We performed an overview of systematic reviews and an updated metanalysis of randomized controlled trials (RCT) assessing the effect of probiotics on Crohn's disease (CD) and ulcerative colitis (UC).
Methods: MEDLINE, Web of Science, and the Cochrane Central Register of Controlled Trials were searched up to September 2023. Primary outcomes were clinical remission and recurrence; secondary outcomes included endoscopic response and remission, and adverse events. We calculated odds ratios (OR) using a random-effects model in R. The quality of systematic reviews was assessed using the AMSTAR-2; the trials' risk of bias was evaluated using the Cochrane Collaboration tool. Evidence certainty was rated using the GRADE framework.
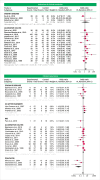
Results: Out of 2613 results, 67 studies (22 systematic reviews and 45 RCTs) met the eligibility criteria. In the updated meta-analysis, the OR for clinical remission in UC and CD was 2.00 (95% CI 1.28-3.11) and 1.61 (95% CI 0.21-12.50), respectively. The subgroup analysis suggested that combining 5-ASA and probiotics may be beneficial for inducing remission in mild-to-moderate UC (OR 2.35, 95% CI 1.29-4.28). Probiotics decreased the odds of recurrence in relapsing pouchitis (OR 0.03, 95% CI 0.00-0.25) and trended toward reducing clinical recurrence in inactive UC (OR 0.65, 95% CI 0.42-1.01). No protective effect against recurrence was identified for CD. Multi-strain formulations appear superior in achieving remission and preventing recurrence in UC. The use of probiotics was not associated with better endoscopic outcomes. Adverse events were similar to control. However, the overall certainty of evidence was low.
Conclusion: Probiotics, particularly multi-strain formulations, appear efficacious for the induction of clinical remission and the prevention of relapse in UC patients as well as for relapsing pouchitis. Notwithstanding, no significant effect was identified for CD. The favorable safety profile of probiotics was also highlighted.
Keywords: Crohn's disease; RCT; clinical outcomes; clinical remission; endoscopic response; inflammatory bowel disease; microbiome modulation; probiotic; recurrence; ulcerative colitis.
© 2024 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
IRL has received financial support for traveling and educational activities or has served as an advisory board member for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Kern, Celltrion, Roche, Ferring, Dr. Falk Pharma, Galapagos, Otsuka Pharmaceutical and Adacyte. MBA has received financial support for traveling and educational activities or has served as an advisory board member for Pfizer, MSD, Takeda, AbbVie, Kern, Janssen, Fresenius Kabi, Biogen, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceuticals, and Tillotts Pharma. FM served as a speaker and received honoraria from Merck Sharp & Dohme, Abbvie, Vifor, Falk, Laboratórios Vitória, Pfizer, Ferring, Hospira, and Biogen. VJ has received consulting/advisory board fees from AbbVie, Alimentiv Inc. [formerly Robarts Clinical Trials], Arena pharmaceuticals, Asieris, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Fresenius Kabi, Galapagos, GlaxoSmithKline, Genetech, Gilead, Janssen, Merck, Mylan, Pandion, Pendopharm, Pfizer, Reistone Biopharma, Roche, Sandoz, Takeda and Topivert; and speaker's fees from, Abbvie, Ferring, Janssen Pfizer Shire and Takeda. Alimentiv, Inc. is an academic gastrointestinal contract research organization (CRO), operating under the Alimentiv Health Trust. Alimentiv, Inc. provides centralized imaging management solutions in clinical trials, including endoscopy, histopathology and magnetic resonance imaging; VJ is a consultant and has neither equity positions nor shares in the corporation. The remaining authors have no conflicts of interest to disclose.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

