U.S. Selected Practice Recommendations for Contraceptive Use, 2024
- PMID: 39106301
- PMCID: PMC11340200
- DOI: 10.15585/mmwr.rr7303a1
U.S. Selected Practice Recommendations for Contraceptive Use, 2024
Abstract
The 2024 U.S. Selected Practice Recommendations for Contraceptive Use (U.S. SPR) addresses a selected group of common, yet sometimes complex, issues regarding initiation and use of specific contraceptive methods. These recommendations for health care providers were updated by CDC after review of the scientific evidence and a meeting with national experts in Atlanta, Georgia, during January 25-27, 2023. The information in this report replaces the 2016 U.S. SPR (CDC. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR 2016;65[No. RR-4]:1-66). Notable updates include 1) updated recommendations for provision of medications for intrauterine device placement, 2) updated recommendations for bleeding irregularities during implant use, 3) new recommendations for testosterone use and risk for pregnancy, and 4) new recommendations for self-administration of injectable contraception. The recommendations in this report are intended to serve as a source of evidence-based clinical practice guidance for health care providers. The goals of these recommendations are to remove unnecessary medical barriers to accessing and using contraception and to support the provision of person-centered contraceptive counseling and services in a noncoercive manner. Health care providers should always consider the individual clinical circumstances of each person seeking contraceptive services. This report is not intended to be a substitute for professional medical advice for individual patients; when needed, patients should seek advice from their health care providers about contraceptive use.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed. To promote transparency, all meeting participants were asked to disclose potential conflicts of interest to CDC before the expert meeting and to report potential conflicts of interest during the introductory portion of the expert meeting. All potential conflicts of interest disclosed by meeting participants are listed. No participants were excluded from discussion based on potential conflicts of interest. CDC staff members who ultimately decided and developed these recommendations have no financial interests or other relationships with the manufacturers of commercial products, suppliers of commercial services, or commercial supporters relevant to these recommendations.
Figures



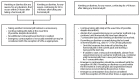

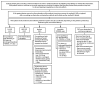
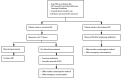
References
-
- CDC. US Public Health Service preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
-
- The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston, MA: Little, Brown and Co; 1994.
-
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the United States. Washington, DC: US Department of Health and Human Services; 2023. https://clinicalinfo.hiv.gov/en/guidelines/perinatal/recommendations-arv...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
