A therapeutic small molecule enhances γ-oscillations and improves cognition/memory in Alzheimer's disease model mice
- PMID: 39106304
- PMCID: PMC11331084
- DOI: 10.1073/pnas.2400420121
A therapeutic small molecule enhances γ-oscillations and improves cognition/memory in Alzheimer's disease model mice
Abstract
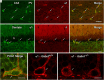
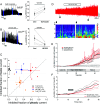
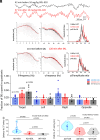
Brain rhythms provide the timing for recruitment of brain activity required for linking together neuronal ensembles engaged in specific tasks. The γ-oscillations (30 to 120 Hz) orchestrate neuronal circuits underlying cognitive processes and working memory. These oscillations are reduced in numerous neurological and psychiatric disorders, including early cognitive decline in Alzheimer's disease (AD). Here, we report on a potent brain-permeable small molecule, DDL-920 that increases γ-oscillations and improves cognition/memory in a mouse model of AD, thus showing promise as a class of therapeutics for AD. We employed anatomical, in vitro and in vivo electrophysiological, and behavioral methods to examine the effects of our lead therapeutic candidate small molecule. As a novel in central nervous system pharmacotherapy, our lead molecule acts as a potent, efficacious, and selective negative allosteric modulator of the γ-aminobutyric acid type A receptors most likely assembled from α1β2δ subunits. These receptors, identified through anatomical and pharmacological means, underlie the tonic inhibition of parvalbumin (PV) expressing interneurons (PV+INs) critically involved in the generation of γ-oscillations. When orally administered twice daily for 2 wk, DDL-920 restored the cognitive/memory impairments of 3- to 4-mo-old AD model mice as measured by their performance in the Barnes maze. Our approach is unique as it is meant to enhance cognitive performance and working memory in a state-dependent manner by engaging and amplifying the brain's endogenous γ-oscillations through enhancing the function of PV+INs.
Keywords: Alzheimer’s disease; GABA-A receptors; gamma oscillations; interneurons; parvalbumin.
Conflict of interest statement
Competing interests statement:Composition and methods for treating neurodgenerative diseases. W.I.P. Organization, ed. (The Regents of the University of California).
Figures

Update of
-
A therapeutic small molecule lead enhances γ-oscillations and improves cognition/memory in Alzheimer's disease model mice.bioRxiv [Preprint]. 2023 Dec 6:2023.12.04.569994. doi: 10.1101/2023.12.04.569994. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Aug 13;121(33):e2400420121. doi: 10.1073/pnas.2400420121. PMID: 38106006 Free PMC article. Updated. Preprint.
References
-
- Couzin-Frankel J., Side effects loom over Alzheimer’s drugs. Science 381, 466–467 (2023). - PubMed
-
- Ives-Deliperi V., Butler J. T., Mechanisms of cognitive impairment in temporal lobe epilepsy: A systematic review of resting-state functional connectivity studies. Epilepsy Behav. 115, 107686 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

