A bioactive supramolecular and covalent polymer scaffold for cartilage repair in a sheep model
- PMID: 39106310
- PMCID: PMC11331086
- DOI: 10.1073/pnas.2405454121
A bioactive supramolecular and covalent polymer scaffold for cartilage repair in a sheep model
Abstract
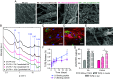
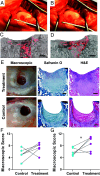
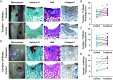
Regeneration of hyaline cartilage in human-sized joints remains a clinical challenge, and it is a critical unmet need that would contribute to longer healthspans. Injectable scaffolds for cartilage repair that integrate both bioactivity and sufficiently robust physical properties to withstand joint stresses offer a promising strategy. We report here on a hybrid biomaterial that combines a bioactive peptide amphiphile supramolecular polymer that specifically binds the chondrogenic cytokine transforming growth factor β-1 (TGFβ-1) and crosslinked hyaluronic acid microgels that drive formation of filament bundles, a hierarchical motif common in natural musculoskeletal tissues. The scaffold is an injectable slurry that generates a porous rubbery material when exposed to calcium ions once placed in cartilage defects. The hybrid material was found to support in vitro chondrogenic differentiation of encapsulated stem cells in response to sustained delivery of TGFβ-1. Using a sheep model, we implanted the scaffold in shallow osteochondral defects and found it can remain localized in mechanically active joints. Evaluation of resected joints showed significantly improved repair of hyaline cartilage in osteochondral defects injected with the scaffold relative to defects injected with the growth factor alone, including implantation in the load-bearing femoral condyle. These results demonstrate the potential of the hybrid biomimetic scaffold as a niche to favor cartilage repair in mechanically active joints using a clinically relevant large-animal model.
Keywords: cartilage regeneration; peptide amphiphiles; self-assembly; supramolecular biomaterials.
Conflict of interest statement
Competing interests statement:J.A.L., N.A.S., M.T.M., and S.I.S. are inventors on patent application WO2023056433A1 filed by Northwestern University. N.A.S. and S.I.S. are stockholders of Amphix Bio, Inc.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

