CD19-Directed CAR T-Cells in a Patient With Refractory MOGAD: Clinical and Immunologic Follow-Up for 1 Year
- PMID: 39106426
- PMCID: PMC11309560
- DOI: 10.1212/NXI.0000000000200292
CD19-Directed CAR T-Cells in a Patient With Refractory MOGAD: Clinical and Immunologic Follow-Up for 1 Year
Abstract
Objectives: In MOG antibody-associated disease (MOGAD), relapse prevention and the treatment approach to refractory symptoms are unknown. We report a patient with refractory MOGAD treated with CD19-directed CAR T-cells.
Methods: CD19-directed CAR T-cells (ARI-0001) were produced in-house by lentiviral transduction of autologous fresh leukapheresis and infused after a conventional lymphodepleting regimen.
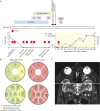
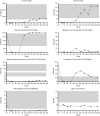
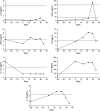
Results: A 18-year-old man developed 2 episodes of myelitis associated with serum MOG-IgG, which were followed by 6 episodes of left optic neuritis (ON) and sustained the presence of MOG-IgG over 6 years despite multiple immunotherapies. After the sixth episode of ON, accompanied by severe residual visual deficits, CAR T-cell treatment was provided without complications. Follow-up of cell counts showed complete depletion of CD19+ B cells at day +7; reconstituted B cells at day +141 showing a naïve B-cell phenotype, and low or absent memory B cells and plasmablasts for 1 year. MOG-IgG titers have remained undetectable since CAR T-cell infusion. The patient had an early episode of left ON at day +29, when MOG-IgG was already negative, and since then he has remained free of relapses without immunotherapy for 1 year.
Discussion: This clinical case shows that CD19-directed CAR T-cell therapy is well-tolerated and is a potential treatment for patients with refractory MOGAD.
Classification of evidence: This provides Class IV evidence. It is a single observational study without controls.
Conflict of interest statement
J.M. Cabrera-Maqueda received speaking honoraria from Sanofi and Bristol-Myers Squibb. M. Sepúlveda received speaking honoraria from Roche, Biogen, and UCB. S. Llufriu received compensation for consulting services and speaker honoraria from Biogen Idec, Novartis, TEVA, Genzyme, Sanofi, Merck, and Bristol-Myers Squibb, and holds grants from the Instituto de Salud Carlos III. E. Martínez-Hernandez received compensation for consulting services and speaker honoraria from Biogen, UCB, Argenx, and Alexion. T. Armangué received personal compensation for speaking fees or consultations, and reimbursement of travel expenses for scientific meetings from Sanofi, Roche, and Alexion. J. Dalmau receives royalties related to autoantibody tests from Athena Diagnostics and Euroimmun Inc. M. Juan collaborates scientifically with the Hospital Clinic spin-off without any return or financial interest. A. Saiz received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Merck, Sanofi, Biogen, Roche, Novartis, Janssen, and Horizon Therapeutics. Y. Blanco received speaking honoraria from Novartis, Roche, Sanofi, Merck, Sandoz, Janssen, and Biogen. The other authors report no disclosures. Go to
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
