Ketogenic diet but not free-sugar restriction alters glucose tolerance, lipid metabolism, peripheral tissue phenotype, and gut microbiome: RCT
- PMID: 39106867
- PMCID: PMC11384946
- DOI: 10.1016/j.xcrm.2024.101667
Ketogenic diet but not free-sugar restriction alters glucose tolerance, lipid metabolism, peripheral tissue phenotype, and gut microbiome: RCT
Abstract
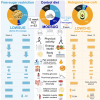
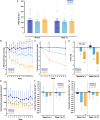
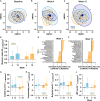
Restricted sugar and ketogenic diets can alter energy balance/metabolism, but decreased energy intake may be compensated by reduced expenditure. In healthy adults, randomization to restricting free sugars or overall carbohydrates (ketogenic diet) for 12 weeks reduces fat mass without changing energy expenditure versus control. Free-sugar restriction minimally affects metabolism or gut microbiome but decreases low-density lipoprotein cholesterol (LDL-C). In contrast, a ketogenic diet decreases glucose tolerance, increases skeletal muscle PDK4, and reduces AMPK and GLUT4 levels. By week 4, the ketogenic diet reduces fasting glucose and increases apolipoprotein B, C-reactive protein, and postprandial glycerol concentrations. However, despite sustained ketosis, these effects are no longer apparent by week 12, when gut microbial beta diversity is altered, possibly reflective of longer-term adjustments to the ketogenic diet and/or energy balance. These data demonstrate that restricting free sugars or overall carbohydrates reduces energy intake without altering physical activity, but with divergent effects on glucose tolerance, lipoprotein profiles, and gut microbiome.
Keywords: body fat; diet; energy balance; energy intake; ketogenic; lipoprotein; low carbohydrate; metabolism; physical activity; sugar.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.D.C. is a co-founder and CTO of SeqBiome Ltd. J.T.G. has received research funding from BBSRC, MRC, Cancer Research UK, the British Heart Foundation, Clasado Biosciences, Lucozade Ribena Suntory, Arla Foods Ingredients, the Cosun Nutrition Center, and the Fruit Juice Science Center; is a scientific advisory board member to ZOE; and has completed paid consultancy for 6d Sports Nutrition, The Dairy Council, PepsiCo, Violicom Medical, Tour Racing Ltd., and SVGC. J.A.B. is an investigator on research grants funded by BBSRC, MRC, the British Heart Foundation, Rare Disease Foundation, EU Hydration Institute, GlaxoSmithKline, Nestlé, Lucozade Ribena Suntory, Arla Foods, Cosun Nutrition Center, American Academy of Sleep Medicine Foundation, and Salus Optima (L3M Technologies Ltd.); has completed paid consultancy for PepsiCo, Kellogg’s, SVGC, and Salus Optima (L3M Technologies Ltd.); is Company Director of Metabolic Solutions Ltd.; receives an annual honorarium as a member of the academic advisory board for the International Olympic Committee Diploma in Sports Nutrition; and receives an annual stipend as Editor-in-Chief of International Journal of Sport Nutrition & Exercise Metabolism.
Figures





References
-
- Scientific Advisory Committee on Nutrition . Public Health England; 2015. Carbohydrates and health report.
-
- World Health Organization Guideline: sugars intake for adults and children. 2015. https://www.who.int/publications/i/item/9789241549028 - PubMed
-
- Scientific Advisory Committee on Nutrition Why 5%? An explanation of SACN’s recommendations about sugars and health. 2015. https://www.gov.uk/government/publications/sacns-sugars-and-health-recom...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

