Stanniocalcin 2 governs cancer cell adaptation to nutrient insufficiency through alleviation of oxidative stress
- PMID: 39107307
- PMCID: PMC11303387
- DOI: 10.1038/s41419-024-06961-7
Stanniocalcin 2 governs cancer cell adaptation to nutrient insufficiency through alleviation of oxidative stress
Abstract
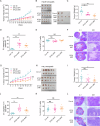
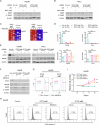
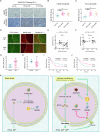
Solid tumours often endure nutrient insufficiency during progression. How tumour cells adapt to temporal and spatial nutrient insufficiency remains unclear. We previously identified STC2 as one of the most upregulated genes in cells exposed to nutrient insufficiency by transcriptome screening, indicating the potential of STC2 in cellular adaptation to nutrient insufficiency. However, the molecular mechanisms underlying STC2 induction by nutrient insufficiency and subsequent adaptation remain elusive. Here, we report that STC2 protein is dramatically increased and secreted into the culture media by Gln-/Glc- deprivation. STC2 promoter contains cis-elements that are activated by ATF4 and p65/RelA, two transcription factors activated by a variety of cellular stress. Biologically, STC2 induction and secretion promote cell survival but attenuate cell proliferation during nutrient insufficiency, thus switching the priority of cancer cells from proliferation to survival. Loss of STC2 impairs tumour growth by inducing both apoptosis and necrosis in mouse xenografts. Mechanistically, under nutrient insufficient conditions, cells have increased levels of reactive oxygen species (ROS), and lack of STC2 further elevates ROS levels that lead to increased apoptosis. RNA-Seq analyses reveal STC2 induction suppresses the expression of monoamine oxidase B (MAOB), a mitochondrial membrane enzyme that produces ROS. Moreover, a negative correlation between STC2 and MAOB levels is also identified in human tumour samples. Importantly, the administration of recombinant STC2 to the culture media effectively suppresses MAOB expression as well as apoptosis, suggesting STC2 functions in an autocrine/paracrine manner. Taken together, our findings indicate that nutrient insufficiency induces STC2 expression, which in turn governs the adaptation of cancer cells to nutrient insufficiency through the maintenance of redox homoeostasis, highlighting the potential of STC2 as a therapeutic target for cancer treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Stanniocalcin 2 governs cancer cell adaptation to nutrient insufficiency through alleviation of oxidative stress.Res Sq [Preprint]. 2024 Feb 27:rs.3.rs-3904465. doi: 10.21203/rs.3.rs-3904465/v1. Res Sq. 2024. Update in: Cell Death Dis. 2024 Aug 6;15(8):567. doi: 10.1038/s41419-024-06961-7. PMID: 38464261 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R01 CA129494/CA/NCI NIH HHS/United States
- CA129494/U.S. Department of Health & Human Services | NIH | Center for Information Technology (Center for Information Technology, National Institutes of Health)
- 82373360/National Natural Science Foundation of China (National Science Foundation of China)
- 2022ZD065/Tianjin Municipal Education Commission
LinkOut - more resources
Full Text Sources

