Green synthesis of magnetite iron oxide nanoparticles using Azadirachta indica leaf extract loaded on reduced graphene oxide and degradation of methylene blue
- PMID: 39107555
- PMCID: PMC11303770
- DOI: 10.1038/s41598-024-69184-y
Green synthesis of magnetite iron oxide nanoparticles using Azadirachta indica leaf extract loaded on reduced graphene oxide and degradation of methylene blue
Abstract
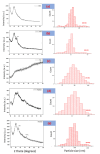
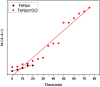
In the current arena, new-generation functional nanomaterials are the key players for smart solutions and applications including environmental decontamination of pollutants. Among the plethora of new-generation nanomaterials, graphene-based nanomaterials and nanocomposites are in the driving seat surpassing their counterparts due to their unique physicochemical characteristics and superior surface chemistry. The purpose of the present research was to synthesize and characterize magnetite iron oxide/reduced graphene oxide nanocomposites (FeNPs/rGO) via a green approach and test its application in the degradation of methylene blue. The modified Hummer's protocol was adopted to synthesize graphene oxide (GO) through a chemical exfoliation approach using a graphitic route. Leaf extract of Azadirachta indica was used as a green reducing agent to reduce GO into reduced graphene oxide (rGO). Then, using the green deposition approach and Azadirachta indica leaf extract, a nanocomposite comprising magnetite iron oxides and reduced graphene oxide i.e., FeNPs/rGO was synthesized. During the synthesis of functionalized FeNPs/rGO, Azadirachta indica leaf extract acted as a reducing, capping, and stabilizing agent. The final synthesized materials were characterized and analyzed using an array of techniques such as scanning electron microscopy (SEM)-energy dispersive X-ray microanalysis (EDX), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction analysis, and UV-visible spectrophotometry. The UV-visible spectrum was used to evaluate the optical characteristics and band gap. Using the FT-IR spectrum, functional groupings were identified in the synthesized graphene-based nanomaterials and nanocomposites. The morphology and elemental analysis of nanomaterials and nanocomposites synthesized via the green deposition process were investigated using SEM-EDX. The GO, rGO, FeNPs, and FeNPs/rGO showed maximum absorption at 232, 265, 395, and 405 nm, respectively. FTIR spectrum showed different functional groups (OH, COOH, C=O), C-O-C) modifying material surfaces. Based on Debye Sherrer's equation, the mean calculated particle size of all synthesized materials was < 100 nm (GO = 60-80, rGO = 90-95, FeNPs = 70-90, Fe/GO = 40-60, and Fe/rGO = 80-85 nm). Graphene-based nanomaterials displayed rough surfaces with clustered and spherical shapes and EDX analysis confirmed the presence of both iron and oxygen in all the nanocomposites. The final nanocomposites produced via the synthetic process degraded approximately 74% of methylene blue. Based on the results, it is plausible to conclude that synthesized FeNPs/rGO nanocomposites can also be used as a potential photocatalyst degrader for other different dye pollutants due to their lower band gap.
Keywords: Azadirachta indica; Graphene-based nanomaterials; Green deposition; Methylene blue; Nanocomposite; Reduced graphene oxide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Roccaro, P., Sgroi, M. & Vagliasindi, F. G. A. Removal of xenobiotic compounds from wastewater for environment protection, treatment processes, and costs. Chem. Eng. Trans.32, 505–510 (2013).
-
- Kanwal, S. et al. Guar gum, Ulva lactuca L. biomass, and xanthan gum-based copolymer novel biosorbent for adsorptive removal of acid orange 10. Biocatal. Agric. Biotech.58, 103173 (2024). 10.1016/j.bcab.2024.103173 - DOI
-
- Mateo-Sagasta, J., Zadeh, S. M., Turral, H., Burke, J. Water pollution from agriculture: a global review. Executive summary. Rome, Italy: FAO; Colombo, Sri Lanka: International Water Management Institute (IWMI). CGIAR Research Program on Water, Land and Ecosystems (WLE), p. 35. (2017)
-
- Zollinger, H. Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments 3rd edn. (Wiley-VCH, Weinheim, Germany, 2003).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

