Hematopoietic stem/progenitor cell transplantation recovers immune defects and prevents lymphomas in Atm-deficient mice
- PMID: 39107850
- PMCID: PMC11302080
- DOI: 10.1186/s40164-024-00544-0
Hematopoietic stem/progenitor cell transplantation recovers immune defects and prevents lymphomas in Atm-deficient mice
Abstract
Background: Ataxia-telangiectasia (A-T) is a rare autosomal recessive multi-system and life-shortening disease, characterized by progressive cerebellar neurodegeneration, immunodeficiency, radiation sensitivity and cancer predisposition, with high incidence of leukemia and lymphoma. A-T is caused by mutations in the gene encoding for ATM protein that has a major role in maintaining the integrity of the genome. Because there are no cures for A-T, we aimed to tackle immunodeficiency and prevent cancer onset/progression by transplantation therapy.
Methods: Enriched hematopoietic stem/progenitor cells (HSPCs), collected from bone marrow of wild-type mice, were transplanted in the caudal vein of 1 month old conditioned Atm-/- mice.
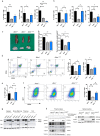
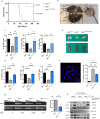
Results: Genomic analyses showed that transplanted Atm positive cells were found in lymphoid organs. B cells isolated from spleen of transplanted mice were able to undergo class switching recombination. Thymocytes were capable to correctly differentiate and consequently an increase of helper T cells and TCRβhi expressing cells was observed. Protein analysis of isolated T and B cells from transplanted mice, revealed that they expressed Atm and responded to DNA damage by initiating an Atm-dependent phosphorylation cascade. Indeed, aberrant metaphases were reduced in transplanted Atm-deficient mice. Six months after transplantation, Atm-/- mice showed signs of aging, but they maintained the rescue of T cells maturation, showed DNA damage response, and prevented thymoma.
Conclusion: We can conclude that wild-type enriched HSPCs transplantation into young Atm-deficient mice can ameliorate A-T hematopoietic phenotypes and prevent tumor of hematopoietic origin.
Keywords: ATM; Genomic stability; HSPCT; Lymphomas; T and B cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

