The ARDS microenvironment enhances MSC-induced repair via VEGF in experimental acute lung inflammation
- PMID: 39108095
- PMCID: PMC11489539
- DOI: 10.1016/j.ymthe.2024.08.003
The ARDS microenvironment enhances MSC-induced repair via VEGF in experimental acute lung inflammation
Abstract
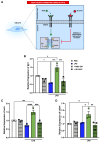
Clinical trials investigating the potential of mesenchymal stromal cells (MSCs) for the treatment of inflammatory diseases, such as acute respiratory distress syndrome (ARDS), have been disappointing, with less than 50% of patients responding to treatment. Licensed MSCs show enhanced therapeutic efficacy in response to cytokine-mediated activation signals. There are two distinct sub-phenotypes of ARDS: hypo- and hyper-inflammatory. We hypothesized that pre-licensing MSCs in a hyper-inflammatory ARDS environment would enhance their therapeutic efficacy in acute lung inflammation (ALI). Serum samples from patients with ARDS were segregated into hypo- and hyper-inflammatory categories based on interleukin (IL)-6 levels. MSCs were licensed with pooled serum from patients with hypo- or hyper-inflammatory ARDS or healthy serum controls. Our findings show that hyper-inflammatory ARDS pre-licensed MSC conditioned medium (MSC-CMHyper) led to a significant enrichment in tight junction expression and enhanced barrier integrity in lung epithelial cells in vitro and in vivo in a vascular endothelial growth factor (VEGF)-dependent manner. Importantly, while both MSC-CMHypo and MSC-CMHyper significantly reduced IL-6 and tumor necrosis factor alpha (TNF-α) levels in the bronchoalveolar lavage fluid (BALF) of lipopolysaccharide (LPS)-induced ALI mice, only MSC-CMHyper significantly reduced lung permeability and overall clinical outcomes including weight loss and clinical score. Thus, the hypo- and hyper-inflammatory ARDS environments may differentially influence MSC cytoprotective and immunomodulatory functions.
Keywords: ARDS; MSC; SARS-CoV-2; VEGF; permeability.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Bernard G.R., Artigas A., Brigham K.L., Carlet J., Falke K., Hudson L., Lamy M., LeGall J.R., Morris A., Spragg R. Report of the American-European consensus conference on ARDS: Definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Med. 1994;20:225–232. - PubMed
-
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium. Barnaby D.P., Becker L.B., Chelico J.D., et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

