Radical Chlorination of Non-Resonant Heterobenzylic C-H Bonds and High-Throughput Diversification of Heterocycles
- PMID: 39108591
- PMCID: PMC11299866
- DOI: 10.1016/j.chempr.2024.04.001
Radical Chlorination of Non-Resonant Heterobenzylic C-H Bonds and High-Throughput Diversification of Heterocycles
Abstract
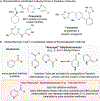
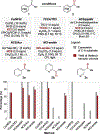
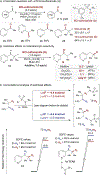
Site-selective functionalization of the heterobenzylic C(sp3)-H bonds of pyridines and related heteroaromatic compounds presents challenges associated with the basic nitrogen atom and the variable reactivity among different positions on the heteroaromatic ring. Methods for functionalization of 2- and 4-alkylpyridines are increasingly available through polar pathways that leverage resonance stabilization of charge build-up at these positions. In contrast, functionalization of 3-alkylpyridines is largely inaccessible. Here, we report a photochemically promoted method for chlorination of non-resonant heterobenzylic C(sp3)-H sites in 3-alkylpyridines and related alkylheteroaromatics. Density functional theory calculations show that the optimal reactivity reflects a balance between the energetics of the two radical-chain propagation steps, with the preferred reagent consisting of an N-chlorosulfonamide. The operationally simple chlorination protocol enables access to heterobenzylic chlorides which serve as versatile intermediates in C-H cross-coupling reactions between heteroaromatic building blocks and diverse oxidatively sensitive nucleophiles using high-throughput experimentation.
Keywords: Chlorination; C–H functionalization; high-throughput experimentation; photochemistry.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures
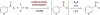



References
-
- Lovering F, Bikker J and Humblet C (2009). Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem, 52, 6752–6756. - PubMed
-
- Cernak T, Dykstra KD, Tyagarajan S, Vachal P and Krska SW (2016). The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev, 45, 546–576. - PubMed
-
- Xue X-S, Ji P, Zhou B and Cheng J-P (2017). The essential role of bond energetics in C–H activation/functionalization. Chem. Rev, 117, 8622–8648. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
