Cxcr4a regulates heart progenitor development and cardiac rhythm in zebrafish
- PMID: 39108621
- PMCID: PMC11300898
- DOI: 10.1016/j.bbrep.2024.101782
Cxcr4a regulates heart progenitor development and cardiac rhythm in zebrafish
Abstract
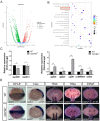
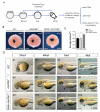
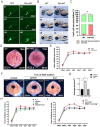
Cxcr4a is involved in multiple organ development including coronary vasculature formation and heart left-right (LR) patterning, whether it is involved in heart progenitor determination and cardiac rhythm regulation is not addressed. Here we showed that in cxcr4a mutants, from 2 days post fertilization (dpf) to 4dpf the embryos transiently displayed pericardial edema and increased cardiac rhythm. While from 5dpf, the heart phenotype disappeared. Detailed analysis demonstrated that, at 36hpf and 48hpf, even though there was no distinct difference in the heart size between cxcr4a mutants and controls, the expression of myl7 was decreased. Further data showed that, the heart progenitors were decreased at 18SS(Somite Stage). Mechanically, RNA-seq, RT-qPCR and in situ experiments showed that the retinoic acid (RA) signaling was upregulated, and the up-regulation of RA signaling may mediate the role of cxcr4a in regulating heart progenitor development. In addition, we also identified that low dose of RA treatment accelerated the cardiac rhythm, being similar to that in cxcr4a mutants. Decreasing RA signaling partially restored the rapid cardiac rhythm in cxcr4a mutants, implying the possibility that RA signaling partially mediates the role of cxcr4a in regulating cardiac rhythm. In conclusion, our study identified cxcr4a simultaneously regulates heart progenitor determination and cardiac rhythm.
Keywords: Cardiac rhythm; Cxcr4a; Heart progenitor; RA signaling; Zebrafish.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Keegan B.R., Meyer D., Yelon D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development. 2004;131(13):3081–3091. - PubMed
-
- Yelon D., Horne S.A., Stainier D.Y. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 1999;214(1):23–37. - PubMed
-
- Veerkamp J., Rudolph F., Cseresnyes Z., Priller F., Otten C., Renz M., Schaefer L., Abdelilah-Seyfried S. Unilateral dampening of Bmp activity by nodal generates cardiac left-right asymmetry. Dev. Cell. 2013;24(6):660–667. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

