Homotrinuclear ruthenium(ii) and rhodium(i) complexes of redox-active tris(ferrocenyl)arene-based tris-phosphanes
- PMID: 39108962
- PMCID: PMC11302318
- DOI: 10.1039/d4ra03822c
Homotrinuclear ruthenium(ii) and rhodium(i) complexes of redox-active tris(ferrocenyl)arene-based tris-phosphanes
Abstract
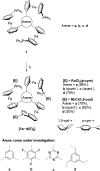
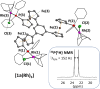
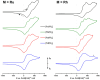
Homotrinuclear complexes of the C 3-symmetric tris(ferrocenyl)arene-based tris-phosphanes 1a-d with ruthenium(ii) ([1a-d(Ru)3]) and rhodium(i) ([1a-d(Rh)3]) were prepared and fully characterised. Complexes [1a-d(Ru)3] and [1a-d(Rh)3] are electrochemically active. The nature of the arene core in 1a-d ranging from benzene, 1,3,5-trifluorobenzene and mesitylene to s-triazine allows to fine-tune the exact oxidation potentials for tailoring the electrochemical response. With a BArF 4 --based supporting electrolyte, a distinct separation of the three iron-centred oxidations of the ligand backbone is observable. Under these conditions, these oxidations are mostly reversible but, especially for the third oxidation, already show signs of irreversibility. In general, while the coordinated metal complex fragment does not strongly alter the electrochemical response of the arene-trisferrocenyl core 1a-d, there are observable differences. Rhodium(i) complexes are oxidised at slightly higher potentials than ruthenium(ii) complexes. In both cases, individual oxidation states for the C6H3(CH2)3-based ligand (1d) are difficult to address and the C3N3-based ligand (1c) shows the most complicated and least reversible electrochemistry with severely broadened third oxidations and reduced reversibility in cyclic voltammetry. The most well-suited system for potential applications in redox-switchable catalysis, in all cases, is the C6H3-based ligand (1a), showing entirely reversible and well-separated redox events.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
LinkOut - more resources
Full Text Sources

