Lipid nanoparticle-encapsulated DOCK11-siRNA efficiently reduces hepatitis B virus cccDNA level in infected mice
- PMID: 39109217
- PMCID: PMC11300937
- DOI: 10.1016/j.omtm.2024.101289
Lipid nanoparticle-encapsulated DOCK11-siRNA efficiently reduces hepatitis B virus cccDNA level in infected mice
Abstract
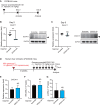
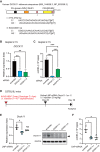
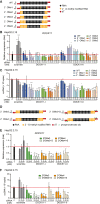
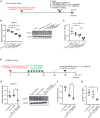
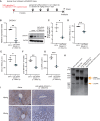
The hepatitis B virus (HBV) infects many people worldwide. As HBV infection frequently leads to liver fibrosis and carcinogenesis, developing anti-HBV therapeutic drugs is urgent. Therapeutic drugs for preventing covalently closed circular DNA (cccDNA) production, which can eliminate HBV infection, are unavailable. The host factor dedicator of cytokinesis 11 (DOCK11) is involved in the synthesis and maintenance of HBV cccDNA in vitro. However, the effectiveness of DOCK11 as a target for the in vivo elimination of HBV cccDNA remains unclear. In this study, we assess whether DOCK11 inhibitors suppress HBV cccDNA production in mouse models of HBV infection. The tocopherol-conjugate hetero- gapmer, a DNA/RNA duplex of gapmer/complementary RNA targeting the DOCK11 sequence, partially reduces the expression of DOCK11, but not that of HBV cccDNA, in the livers of HBV-infected human hepatocyte chimeric mice, along with weight loss and decreased serum human albumin levels. Lipid nanoparticle-encapsulated chemically modified siRNAs specific for DOCK11 suppress DOCK11 expression and decrease HBV cccDNA levels without adverse effects in the mice. Therefore, nucleic acid-based drugs targeting DOCK11 in hepatocytes are potentially effective anti-HBV therapeutics that can reduce HBV cccDNA levels in vivo.
Keywords: DOCK11; HBV; LNP-siRNA; cccDNA; gapmer; hetero gapmer; nucleic acid medicine.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

