Actin networks modulate heterogeneous NF-κB dynamics in response to TNFα
- PMID: 39110005
- PMCID: PMC11524587
- DOI: 10.7554/eLife.86042
Actin networks modulate heterogeneous NF-κB dynamics in response to TNFα
Abstract
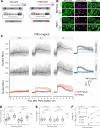
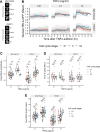
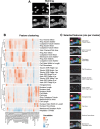
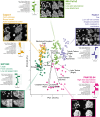
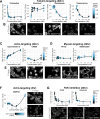
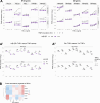
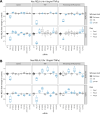
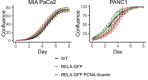
The canonical NF-κB transcription factor RELA is a master regulator of immune and stress responses and is upregulated in pancreatic ductal adenocardinoma (PDAC) tumours. In this study, we characterised previously unexplored endogenous RELA-GFP dynamics in PDAC cell lines through live single-cell imaging. Our observations revealed that TNFα stimulation induces rapid, sustained, and non-oscillatory nuclear translocation of RELA. Through Bayesian analysis of single-cell datasets with variation in nuclear RELA, we predicted that RELA heterogeneity in PDAC cell lines is dependent on F-actin dynamics. RNA-seq analysis identified distinct clusters of RELA-regulated gene expression in PDAC cells, including TNFα-induced RELA upregulation of the actin regulators NUAK2 and ARHGAP31. Further, siRNA-mediated depletion of ARHGAP31 and NUAK2 altered TNFα-stimulated nuclear RELA dynamics in PDAC cells, establishing a novel negative feedback loop that regulates RELA activation by TNFα. Additionally, we characterised the NF-κB pathway in PDAC cells, identifying how NF-κB/IκB proteins genetically and physically interact with RELA in the absence or presence of TNFα. Taken together, we provide computational and experimental support for interdependence between the F-actin network and the NF-κB pathway with RELA translocation dynamics in PDAC.
Keywords: NF-κB; PDAC; RELA; TNF; actin; cancer biology; cell biology; human; single-cell dynamics.
Plain language summary
The prognosis for the most common type of pancreatic cancer, pancreatic ductal adenocarcinoma, also known as PDAC, remains poor. Only around 4% of PDAC patients are likely to live 5 years after being diagnosed. The immune system plays a part in the progression of PDAC, as increased inflammation contributes to the growth of the tumour and its ability to resist treatment. The NF-κB proteins of the immune system are transcription factors that control when and how much certain other proteins are produced. The protein RELA is an important member of the NF-κB family. It is known to be overactivated in PDAC tumours and may be responsible for the high levels of inflammation found in this type of pancreatic cancer. A better understanding of how RELA is activated in PDAC cells will help develop medicines targeting this process. Butera et al. combined experimental and computational approaches to model a network of interactions between key molecules in lines of human PDAC cells grown in the laboratory to investigate how RELA is controlled. Usually, when the RELA protein is activated, it is located in the cell’s nucleus, this means that tracking the location of RELA within the cell can reveal its activated form. To follow RELA, it was labelled with a fluorescent marker and visualised using live, high-throughput fluorescence microscopy. When RELA was activated with a pro-inflammatory molecule TNFα, it changed how it moved in and out of the cell’s nucleus. Using computational modelling approaches, Butera et al. could build a statistical model that revealed that the location and activity of RELA is affected by actin, a key component of the cell’s molecular scaffolding called the cytoskeleton. When actin was disrupted, RELA's activity was altered. Moreover, profiling targets of RELA using RNA sequencing revealed two genes that encode proteins related to the regulation of actin. This indicates that RELA activity – which is itself affected by actin organisation – can feed back to regulate actin. Butera et al. have identified genes regulated by RELA in PDAC cells that could be used as targets for anti-cancer drug development. Further research into the feedback between RELA and actin will help untangle the complex network that causes inflammation in pancreatic cancer.
© 2024, Butera et al.
Conflict of interest statement
FB, JS, LD, CB No competing interests declared
Figures






Update of
- doi: 10.1101/2022.01.19.476961
Similar articles
-
Therapeutic targeting of FOSL1 and RELA-dependent transcriptional mechanisms to suppress pancreatic cancer metastasis.Cell Death Dis. 2025 Jul 9;16(1):504. doi: 10.1038/s41419-025-07810-x. Cell Death Dis. 2025. PMID: 40634284 Free PMC article.
-
RELA/NEAT1/miR-302a-3p/RELA feedback loop modulates pancreatic ductal adenocarcinoma cell proliferation and migration.J Cell Physiol. 2019 Apr;234(4):3583-3597. doi: 10.1002/jcp.27039. Epub 2018 Oct 26. J Cell Physiol. 2019. PMID: 30362505
-
c-Rel is a critical mediator of NF-κB-dependent TRAIL resistance of pancreatic cancer cells.Cell Death Dis. 2014 Oct 9;5(10):e1455. doi: 10.1038/cddis.2014.417. Cell Death Dis. 2014. PMID: 25299780 Free PMC article.
-
Advancement of NF-κB Signaling Pathway: A Novel Target in Pancreatic Cancer.Int J Mol Sci. 2018 Dec 5;19(12):3890. doi: 10.3390/ijms19123890. Int J Mol Sci. 2018. PMID: 30563089 Free PMC article. Review.
-
NF-kappaB function in the human myometrium during pregnancy and parturition.Histol Histopathol. 2010 Jul;25(7):945-56. doi: 10.14670/HH-25.945. Histol Histopathol. 2010. PMID: 20503182 Review.
Cited by
-
Variation in bulk RNA-seq and estimated cell type proportion using deconvolution when comparing pancreatic cancer samples within the same individual.medRxiv [Preprint]. 2025 May 6:2025.05.05.25326976. doi: 10.1101/2025.05.05.25326976. medRxiv. 2025. PMID: 40385431 Free PMC article. Preprint.
-
The PARP inhibitor talazoparib synergizes with reovirus to induce cancer killing and tumour control in vivo in mouse models.Nat Commun. 2025 Jul 8;16(1):6299. doi: 10.1038/s41467-025-61297-w. Nat Commun. 2025. PMID: 40628717 Free PMC article.
References
-
- Alraies Z, Rivera CA, Delgado M-G, Sanséau D, Maurin M, Amadio R, Maria Piperno G, Dunsmore G, Yatim A, Lacerda Mariano L, Kniazeva A, Calmettes V, Sáez PJ, Williart A, Popard H, Gratia M, Lamiable O, Moreau A, Fusilier Z, Crestey L, Albaud B, Legoix P, Dejean AS, Le Dorze A-L, Nakano H, Cook DN, Lawrence T, Manel N, Benvenuti F, Ginhoux F, Moreau HD, P F Nader G, Piel M, Lennon-Duménil A-M. Cell shape sensing licenses dendritic cells for homeostatic migration to lymph nodes. Nature Immunology. 2024;25:1193–1206. doi: 10.1038/s41590-024-01856-3. - DOI - PMC - PubMed
-
- Ankers JM, Awais R, Jones NA, Boyd J, Ryan S, Adamson AD, Harper CV, Bridge L, Spiller DG, Jackson DA, Paszek P, Sée V, White MR. Dynamic NF-κB and E2F interactions control the priority and timing of inflammatory signalling and cell proliferation. eLife. 2016;5:e10473. doi: 10.7554/eLife.10473. - DOI - PMC - PubMed
-
- Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, Ryan S, Spiller DG, Unitt JF, Broomhead DS, Kell DB, Rand DA, Sée V, White MRH. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science. 2009;324:242–246. doi: 10.1126/science.1164860. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- C37275/Cancer Research UK supported by Stand Up to Cancer UK
- A20146/Cancer Research UK supported by Stand Up to Cancer UK
- S_3567/CRUK_/Cancer Research UK/United Kingdom
- C37275/Cancer Research UK (CRUK) supported by Stand Up to Cancer UK
- A20146/Cancer Research UK (CRUK) supported by Stand Up to Cancer UK
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

