Defective autophagy of pericytes enhances radiation-induced senescence promoting radiation brain injury
- PMID: 39110121
- PMCID: PMC11630511
- DOI: 10.1093/neuonc/noae153
Defective autophagy of pericytes enhances radiation-induced senescence promoting radiation brain injury
Abstract
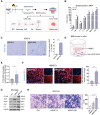
Background: Radiation-induced brain injury (RBI) represents a major challenge for cancer patients undergoing cranial radiotherapy. However, the molecular mechanisms and therapeutic strategies of RBI remain inconclusive. With the continuous exploration of the mechanisms of RBI, an increasing number of studies have implicated cerebrovascular dysfunction as a key factor in RBI-related cognitive impairment. As pericytes are a component of the neurovascular unit, there is still a lack of understanding in current research about the specific role and function of pericytes in RBI.
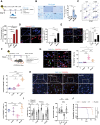
Methods: We constructed a mouse model of RBI-associated cognitive dysfunction in vivo and an in vitro radiation-induced pericyte model to explore the effects of senescent pericytes on the blood-brain barrier (BBB) and normal central nervous system cells, even glioma cells. To further clarify the effects of pericyte autophagy on senescence, molecular mechanisms were explored at the animal and cellular levels. Finally, we validated the clearance of pericyte senescence by using a senolytic drug and all-trans retinoic acid to investigate the role of radiation-induced pericyte senescence.
Results: Our findings indicated that radiation-induced pericyte senescence plays a key role in BBB dysfunction, leading to RBI and subsequent cognitive decline. Strikingly, pericyte senescence also contributed to the growth and invasion of glioma cells. We further demonstrated that defective autophagy in pericytes is a vital regulatory mechanism for pericyte senescence. Moreover, autophagy activated by rapamycin could reverse pericyte senescence. Notably, the elimination of senescent cells by senolytic drugs significantly mitigated radiation-induced cognitive dysfunction.
Conclusions: Our results demonstrated that pericyte senescence may be a promising therapeutic target for RBI and glioma progression.
Keywords: autophagy; cellular senescence; pericyte; radiation-induced brain injury.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

