A Real-World Comparison of Clinical Effectiveness in Patients with Rheumatoid Arthritis Treated with Upadacitinib, Tumor Necrosis Factor Inhibitors, and Other Advanced Therapies After Switching from an Initial Tumor Necrosis Factor Inhibitor
- PMID: 39110310
- PMCID: PMC11349780
- DOI: 10.1007/s12325-024-02948-0
A Real-World Comparison of Clinical Effectiveness in Patients with Rheumatoid Arthritis Treated with Upadacitinib, Tumor Necrosis Factor Inhibitors, and Other Advanced Therapies After Switching from an Initial Tumor Necrosis Factor Inhibitor
Abstract
Introduction: This study compared the clinical effectiveness of switching from tumor necrosis factor inhibitor (TNFi) to upadacitinib (TNFi-UPA), another TNFi (TNFi-TNFi), or an advanced therapy with another mechanism of action (TNFi-other MOA) in patients with rheumatoid arthritis (RA).
Methods: Data were drawn from the Adelphi RA Disease Specific Programme™, a cross-sectional survey administered to rheumatologists and their consulting patients in Germany, France, Italy, Spain, the UK, Japan, Canada, and the USA from May 2021 to January 2022. Patients who switched treatment from an initial TNFi were stratified by subsequent therapy of interest: TNFi-UPA, TNFi-TNFi, or TNFi-other MOA. Physician-reported clinical outcomes including disease activity (with formal DAS28 scoring available for 29% of patients) categorized as remission, low/moderate/high disease activity, as well as pain were recorded at initiation of current treatment and ≥ 6 months from treatment switch. Fatigue and treatment adherence were measured ≥ 6 months from treatment switch. Inverse-probability-weighted regression adjustment compared outcomes by subsequent class of therapy: TNFi-UPA versus TNFi-TNFi, or TNFi-UPA versus TNFi-other MOA.
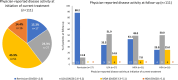
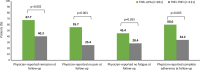
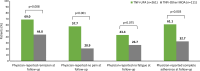
Results: Of 503 patients who switched from their first TNFi, 261 were in TNFi-UPA, 128 in TNFi-TNFi, and 114 in TNFi-other MOA groups. At the time of switch, most patients had moderate/high disease activity (TNFi-UPA: 73%; TNFi-TNFi: 52%; TNFi-other MOA: 60%). After adjustment for differences in characteristics at point of switch, patients in TNFi-UPA group (n = 261) were significantly more likely to achieve physician-reported remission (67.7% vs. 40.3%; p = 0.0015), no pain (55.7% vs. 25.4%; p = 0.0007), and complete adherence (60.0% vs. 34.2%; p = 0.0049) compared with patients in TNFi-TNFi group (n = 121). Similar findings were observed for TNFi-UPA versus TNFi-other MOA groups (n = 111).
Conclusion: Patients who switched from TNFi to UPA had significantly better clinical outcomes of remission, no pain, and complete adherence than those who cycled TNFi or switched to another MOA.
Keywords: Cycling; Real-world; Rheumatoid arthritis; Switching; Tumor necrosis factor inhibitor; Upadacitinib.
© 2024. The Author(s).
Conflict of interest statement
Roberto Caporali has received speaker and/or consultant fees from AbbVie, Accord, Celltrion, Eli Lilly, Fresenius, Galapagos, Janssen, MSD, Novartis, Pfizer Inc, and UCB. Peter C. Taylor has received grant and/or research support from Galapagos and has acted as a consultant for AbbVie, Biogen, Eli Lilly, Fresenius, Galapagos, Gilead Sciences, GlaxoSmithKline, Janssen, Nordic Pharma, Pfizer Inc, Sanofi, and UCB. Jayesh Patel, Aditi Kadakia, and Sander Strengholt are employees of AbbVie Inc and may own stock. Oliver Howell, Jack Milligan, and Sophie Barlow are employees of Adelphi Real World, who acted as consultants to AbbVie for this analysis and have no other conflicts of interest.
Figures


References
-
- Chauhan K, Jandu JS, Brent LH, Al-Dhahir MA. Rheumatoid arthritis. Treasure Island (FL): StatPearls; 2023. - PubMed
-
- Kekow J, Moots R, Khandker R, Melin J, Freundlich B, Singh A. Improvements in patient-reported outcomes, symptoms of depression and anxiety, and their association with clinical remission among patients with moderate-to-severe active early rheumatoid arthritis. Rheumatology. 2011;50(2):401–9. 10.1093/rheumatology/keq327 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

