A Vaping Cessation Text Message Program for Adolescent E-Cigarette Users: A Randomized Clinical Trial
- PMID: 39110436
- PMCID: PMC11307165
- DOI: 10.1001/jama.2024.11057
A Vaping Cessation Text Message Program for Adolescent E-Cigarette Users: A Randomized Clinical Trial
Erratum in
-
Incorrect Description of Adverse Childhood Experiences Scoring.JAMA. 2025 Mar 18;333(11):1007. doi: 10.1001/jama.2025.1393. JAMA. 2025. PMID: 39976989 Free PMC article. No abstract available.
Abstract
Importance: E-cigarettes are the most commonly used tobacco product among adolescents. Despite known harms of nicotine exposure among teens, there are no empirically tested vaping cessation interventions.
Objective: To compare the effectiveness of a text message program for nicotine vaping cessation among adolescents with assessment-only control.
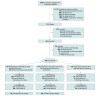
Design, setting, and participants: A parallel, 2-group, double-blind, individually randomized clinical trial with follow-ups at 1 and 7 months after randomization was conducted from October 1, 2021, to October 18, 2023. Participants were recruited via social media ads; the intervention was delivered via text message; and assessments were completed online or by telephone. Eligible individuals were US residents aged 13 to 17 years who reported past 30-day e-cigarette use, were interested in quitting within 30 days, and owned a mobile phone with an active text message plan. To optimize study retention, all participants received monthly assessments via text message about e-cigarette use.
Interventions: Assessment-only controls (n = 744) received only study retention text messages. Intervention participants (n = 759) also received an automated, interactive text message program for vaping cessation that delivers cognitive and behavioral coping skills training and social support.
Main outcomes and measures: The primary outcome was self-reported 30-day point-prevalence abstinence from vaping at 7 months analyzed as intention-to-treat, with missingness coded as vaping.
Results: Among n = 1503 adolescents randomized, average age was 16.4 (SD, 0.8) years. The sample was 50.6% female, 42.1% male, and 7.4% nonbinary/other; 10.2% Black/African American, 62.6% White, 18.5% multiracial, and 8.7% another race; 16.2% Hispanic; 42.5% sexual minority; and 76.2% vaped within 30 minutes of waking. The 7-month follow-up rate was 70.8%. Point-prevalence abstinence rates were 37.8% (95% CI, 34.4%-41.3%) among intervention participants and 28.0% (95% CI, 24.9%-31.3%) among control participants (relative risk, 1.35 [95% CI, 1.17-1.57]; P < .001). No baseline variables moderated the treatment-outcome relationship. There was no evidence that adolescents who quit vaping transitioned to combustible tobacco products.
Conclusions and relevance: A tailored, interactive text message intervention increased self-reported vaping cessation rates among adolescents recruited via social media channels.
Trial registration: ClinicalTrials.gov Identifier: NCT04919590.
Conflict of interest statement
Figures
Comment in
-
Supporting Adolescents' Desire to Quit E-Cigarettes.JAMA. 2024 Sep 3;332(9):711-712. doi: 10.1001/jama.2024.13142. JAMA. 2024. PMID: 39110453 No abstract available.
-
E-Cigarette Use in Adolescents and Adults-A JAMA Collection.JAMA. 2024 Sep 3;332(9):709-710. doi: 10.1001/jama.2024.15912. JAMA. 2024. PMID: 39110585 No abstract available.
References
-
- National Cancer Institute . Online summary of trends in US cancer control measures: youth tobacco use. US Department of Health and Human Services. Updated August 2023. Accessed November 25, 2023. https://progressreport.cancer.gov/prevention/youth_smoking
-
- US Department of Health and Human Services, Office of Surgeon General . Surgeon General’s Advisory on E-Cigarette Use Among Youth. Published December 2018. Accessed April 1, 2024. https://e-cigarettes.surgeongeneral.gov/documents/surgeon-generals-advis...
-
- National Academies of Sciences Engineering and Medicine . Public Health Consequences of E-Cigarettes. National Academies Press; 2018. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

