Neural pathways associated with reduced rigidity during pallidal deep brain stimulation for Parkinson's disease
- PMID: 39110516
- PMCID: PMC11427047
- DOI: 10.1152/jn.00155.2024
Neural pathways associated with reduced rigidity during pallidal deep brain stimulation for Parkinson's disease
Abstract
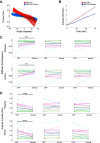
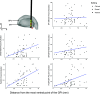
Deep brain stimulation (DBS) of the internal segment of the globus pallidus (GPi) can markedly reduce muscle rigidity in people with Parkinson's disease (PD); however, the mechanisms mediating this effect are poorly understood. Computational modeling of DBS provides a method to estimate the relative contributions of neural pathway activations to changes in outcomes. In this study, we generated subject-specific biophysical models of GPi DBS (derived from individual 7-T MRI), including pallidal efferent, putamenal efferent, and internal capsule pathways, to investigate how activation of neural pathways contributed to changes in forearm rigidity in PD. Ten individuals (17 arms) were tested off medication under four conditions: off stimulation, on clinically optimized stimulation, and on stimulation specifically targeting the dorsal GPi or ventral GPi. Quantitative measures of forearm rigidity, with and without a contralateral activation maneuver, were obtained with a robotic manipulandum. Clinically optimized GPi DBS settings significantly reduced forearm rigidity (P < 0.001), which aligned with GPi efferent fiber activation. The model demonstrated that GPi efferent axons could be activated at any location along the GPi dorsal-ventral axis. These results provide evidence that rigidity reduction produced by GPi DBS is mediated by preferential activation of GPi efferents to the thalamus, likely leading to a reduction in excitability of the muscle stretch reflex via overdriving pallidofugal output.NEW & NOTEWORTHY Subject-specific computational models of pallidal deep brain stimulation, in conjunction with quantitative measures of forearm rigidity, were used to examine the neural pathways mediating stimulation-induced changes in rigidity in people with Parkinson's disease. The model uniquely included internal, efferent and adjacent pathways of the basal ganglia. The results demonstrate that reductions in rigidity evoked by deep brain stimulation were principally mediated by the activation of globus pallidus internus efferent pathways.
Keywords: Parkinson’s disease; computational model; deep brain stimulation; globus pallidus; rigidity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








References
Publication types
MeSH terms
Grants and funding
- S10 OD025256/CD/ODCDC CDC HHS/United States
- P50 NS098573/NS/NINDS NIH HHS/United States
- F31 HD112-86-01A/HHS | National Institutes of Health (NIH)
- S10 OD025256/OD/NIH HHS/United States
- DGE-1734815/Neurosciences Foundation (NSF)
- RO1 NS085188/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- F31 HD112086/HD/NICHD NIH HHS/United States
- P50 NS123109/NS/NINDS NIH HHS/United States
- R01 NS124814/NS/NINDS NIH HHS/United States
- Minnesota Discovery Research Innovation Economy (MnDrive)
- R01NS124814/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS085188/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

