The first high-altitude autotetraploid haplotype-resolved genome assembled (Rhododendron nivale subsp. boreale) provides new insights into mountaintop adaptation
- PMID: 39110622
- PMCID: PMC11304948
- DOI: 10.1093/gigascience/giae052
The first high-altitude autotetraploid haplotype-resolved genome assembled (Rhododendron nivale subsp. boreale) provides new insights into mountaintop adaptation
Abstract
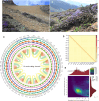
Background: Rhododendron nivale subsp. boreale Philipson et M. N. Philipson is an alpine woody species with ornamental qualities that serve as the predominant species in mountainous scrub habitats found at an altitude of ∼4,200 m. As a high-altitude woody polyploid, this species may serve as a model to understand how plants adapt to alpine environments. Despite its ecological significance, the lack of genomic resources has hindered a comprehensive understanding of its evolutionary and adaptive characteristics in high-altitude mountainous environments.
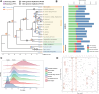
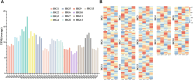
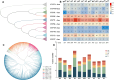
Findings: We sequenced and assembled the genome of R. nivale subsp. boreale, an assembly of the first subgenus Rhododendron and the first high-altitude woody flowering tetraploid, contributing an important genomic resource for alpine woody flora. The assembly included 52 pseudochromosomes (scaffold N50 = 42.93 Mb; BUSCO = 98.8%; QV = 45.51; S-AQI = 98.69), which belonged to 4 haplotypes, harboring 127,810 predicted protein-coding genes. Conjoint k-mer analysis, collinearity assessment, and phylogenetic investigation corroborated autotetraploid identity. Comparative genomic analysis revealed that R. nivale subsp. boreale originated as a neopolyploid of R. nivale and underwent 2 rounds of ancient polyploidy events. Transcriptional expression analysis showed that differences in expression between alleles were common and randomly distributed in the genome. We identified extended gene families and signatures of positive selection that are involved not only in adaptation to the mountaintop ecosystem (response to stress and developmental regulation) but also in autotetraploid reproduction (meiotic stabilization). Additionally, the expression levels of the (group VII ethylene response factor transcription factors) ERF VIIs were significantly higher than the mean global gene expression. We suspect that these changes have enabled the success of this species at high altitudes.
Conclusions: We assembled the first high-altitude autopolyploid genome and achieved chromosome-level assembly within the subgenus Rhododendron. In addition, a high-altitude adaptation strategy of R. nivale subsp. boreale was reasonably speculated. This study provides valuable data for the exploration of alpine mountaintop adaptations and the correlation between extreme environments and species polyploidization.
Keywords: Rhododendron; autotetraploid; evolutionary history; harsh environment; mountaintop adaptation.
© The Author(s) 2024. Published by Oxford University Press GigaScience.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Deciphering the complex organelle genomes of two Rhododendron species and insights into adaptive evolution patterns in high-altitude.BMC Plant Biol. 2024 Nov 8;24(1):1054. doi: 10.1186/s12870-024-05761-7. BMC Plant Biol. 2024. PMID: 39511517 Free PMC article.
-
High-quality evergreen azalea genome reveals tandem duplication-facilitated low-altitude adaptability and floral scent evolution.Plant Biotechnol J. 2021 Dec;19(12):2544-2560. doi: 10.1111/pbi.13680. Epub 2021 Aug 21. Plant Biotechnol J. 2021. PMID: 34375461 Free PMC article.
-
The chromosome-scale genome assembly, annotation and evolution of Rhododendron henanense subsp. lingbaoense.Mol Ecol Resour. 2022 Apr;22(3):988-1001. doi: 10.1111/1755-0998.13529. Epub 2021 Oct 25. Mol Ecol Resour. 2022. PMID: 34652864
-
Polyploidy and interspecific hybridization: partners for adaptation, speciation and evolution in plants.Ann Bot. 2017 Aug 1;120(2):183-194. doi: 10.1093/aob/mcx079. Ann Bot. 2017. PMID: 28854567 Free PMC article. Review.
-
The ancient wave of polyploidization events in flowering plants and their facilitated adaptation to environmental stress.Plant Cell Environ. 2020 Dec;43(12):2847-2856. doi: 10.1111/pce.13898. Epub 2020 Oct 13. Plant Cell Environ. 2020. PMID: 33001478 Review.
Cited by
-
Adaptation of High-Altitude Plants to Harsh Environments: Application of Phenotypic-Variation-Related Methods and Multi-Omics Techniques.Int J Mol Sci. 2024 Nov 26;25(23):12666. doi: 10.3390/ijms252312666. Int J Mol Sci. 2024. PMID: 39684378 Free PMC article. Review.
-
T2T genome, pan-genome analysis, and heat stress response genes in Rhododendron species.Imeta. 2025 Mar 5;4(2):e70010. doi: 10.1002/imt2.70010. eCollection 2025 Apr. Imeta. 2025. PMID: 40236772 Free PMC article.
References
-
- Fang R, Min TL. The floristic study on the genus Rhododendron. Acta Botanica Yunnanica. 1995;17:359–79.. https://journal.kib.ac.cn/EN/Y1995/V17/I04/1.
-
- Chen YS, Deng T, Zhou Z, et al. Is the East Asian flora ancient or not?. Natl Sci Rev. 2018;5:920–32. 10.1093/nsr/nwx156. - DOI
-
- Basnett S, Rengaian G. A comprehensive review on the taxonomy, ecology, reproductive biology, economic importance and conservation status of Indian Himalayan Rhododendrons. Bot Rev. 2022;88:505–44. 10.1007/s12229-021-09273-z. - DOI
-
- Darlington CD, Wylie AP. Chromosome Atlas of Flowering Plants. George Allen and Unwin: London, UK; 1955: 217–18.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

