A self-amplifying RNA vaccine prevents enterovirus D68 infection and disease in preclinical models
- PMID: 39110777
- PMCID: PMC11789928
- DOI: 10.1126/scitranslmed.adi1625
A self-amplifying RNA vaccine prevents enterovirus D68 infection and disease in preclinical models
Abstract
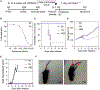
The recent emergence and rapid response to severe acute respiratory syndrome coronavirus 2 was enabled by prototype pathogen and vaccine platform approaches, driven by the preemptive application of RNA vaccine technology to the related Middle East respiratory syndrome coronavirus. Recently, the National Institutes of Allergy and Infectious Diseases identified nine virus families of concern, eight enveloped virus families and one nonenveloped virus family, for which vaccine generation is a priority. Although RNA vaccines have been described for a variety of enveloped viruses, a roadmap for their use against nonenveloped viruses is lacking. Enterovirus D68 was recently designated a prototype pathogen within the family Picornaviridae of nonenveloped viruses because of its rapid evolution and respiratory route of transmission, coupled with a lack of diverse anti-enterovirus vaccine approaches in development. Here, we describe a proof-of-concept approach using a clinical stage RNA vaccine platform that induced robust enterovirus D68-neutralizing antibody responses in mice and nonhuman primates and prevented upper and lower respiratory tract infections and neurological disease in mice. In addition, we used our platform to rapidly characterize the antigenic diversity within the six genotypes of enterovirus D68, providing the necessary data to inform multivalent vaccine compositions that can elicit optimal breadth of neutralizing responses. These results demonstrate that RNA vaccines can be used as tools in our pandemic-preparedness toolbox for nonenveloped viruses.
Conflict of interest statement
N.L.W., S.P., T.K., A.S., J.H.E., A.P.K., J.A., and P.B. have equity interest in HDT Bio. J.H.E., A.P.K., J.A., T.K., and P.B. are coinventors on patents (US Patent nos. 11,458,209; 11,433,142; 11,752,218; 11,648,321; and 11,654,200) and patent applications (PCT/US22/76787, PCT/US23/60225, and PCT/US2024/010326) pertaining to the LION formulation and repRNA compositions described in the studies. M.R.V. is coinventor on a patent application (PCT/US2020/043415) for anti–EV-D68 human monoclonal antibodies, received a contract from HDT Bio to support this work, and previously consulted for HDT Bio. The other authors declare that they have no competing interests.
Figures





References
-
- Meyers L, Dien Bard J, Galvin B, Nawrocki J, Niesters HGM, Stellrecht KA, St. George K, Daly JA, Blaschke AJ, Robinson C, Wang H, Cook CV, Hassan F, Dominguez SR, Pretty K, Naccache S, Olin KE, Althouse BM, Jones JD, Ginocchio CC, Poritz MA, Leber A, Selvarangan R, Enterovirus D68 outbreak detection through a syndromic disease epidemiology network. J. Clin. Virol 124, 104262 (2020). - PubMed
-
- Cortese MM, Kambhampati AK, Schuster JE, Alhinai Z, Nelson GR, Guzman Perez-Carrillo GJ, Vossough A, Smit MA, McKinstry RC, Zinkus T, Moore KR, Rogg JM, Candee MS, Sejvar JJ, Hopkins SE, A ten-year retrospective evaluation of acute flaccid myelitis at 5 pediatric centers in the United States, 2005–2014. PLOS ONE 15, 2005–2014 (2020). - PMC - PubMed
-
- Greninger AL, Naccache SN, Messacar K, Clayton A, Yu G, Somasekar S, Federman S, Stryke D, Anderson C, Yagi S, Messenger S, Wadford D, Xia D, Watt JP, Van Haren K, Dominguez SR, Glaser C, Aldrovandi G, Chiu CY, A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA: A retrospective cohort study. Lancet Infect. Dis 15, 671–682 (2015). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

