Insulin restores retinal ganglion cell functional connectivity and promotes visual recovery in glaucoma
- PMID: 39110798
- PMCID: PMC11305393
- DOI: 10.1126/sciadv.adl5722
Insulin restores retinal ganglion cell functional connectivity and promotes visual recovery in glaucoma
Abstract
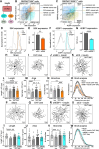
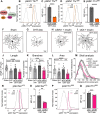
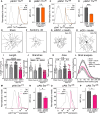
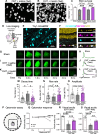
Dendrite pathology and synaptic loss result in neural circuit dysfunction, a common feature of neurodegenerative diseases. There is a lack of strategies that target dendritic and synaptic regeneration to promote neurorecovery. We show that daily human recombinant insulin eye drops stimulate retinal ganglion cell (RGC) dendrite and synapse regeneration during ocular hypertension, a risk factor to develop glaucoma. We demonstrate that the ribosomal protein p70S6 kinase (S6K) is essential for insulin-dependent dendritic regrowth. Furthermore, S6K phosphorylation of the stress-activated protein kinase-interacting protein 1 (SIN1), a link between the mammalian target of rapamycin complexes 1 and 2 (mTORC1/2), is required for insulin-induced dendritic regeneration. Using two-photon microscopy live retinal imaging, we show that insulin rescues single-RGC light-evoked calcium (Ca2+) dynamics. We further demonstrate that insulin enhances neuronal survival and retina-brain connectivity leading to improved optomotor reflex-elicited behaviors. Our data support that insulin is a compelling pro-regenerative strategy with potential clinical implications for the treatment and management of glaucoma.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

