Long-Term Dementia Risk in Parkinson Disease
- PMID: 39110916
- PMCID: PMC11318527
- DOI: 10.1212/WNL.0000000000209699
Long-Term Dementia Risk in Parkinson Disease
Abstract
Background and objectives: It is widely cited that dementia occurs in up to 80% of patients with Parkinson disease (PD), but studies reporting such high rates were published over two decades ago, had relatively small samples, and had other limitations. We aimed to determine long-term dementia risk in PD using data from two large, ongoing, prospective, observational studies.
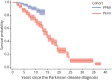
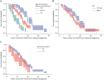
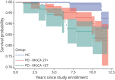
Methods: Participants from the Parkinson's Progression Markers Initiative (PPMI), a multisite international study, and a long-standing PD research cohort at the University of Pennsylvania (Penn), a single site study at a tertiary movement disorders center, were recruited. PPMI enrolled de novo, untreated PD participants and Penn a convenience cohort from a large clinical center. For PPMI, a cognitive battery is administered annually, and a site investigator makes a cognitive diagnosis. At Penn, a comprehensive cognitive battery is administered either annually or biennially, and a cognitive diagnosis is made by expert consensus. Interval-censored survival curves were fit for time from PD diagnosis to stable dementia diagnosis for each cohort, using cognitive diagnosis of dementia as the primary end point and Montreal Cognitive Assessment (MoCA) score <21 and Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) Part I cognition score ≥3 as secondary end points for PPMI. In addition, estimated dementia probability by PD disease duration was tabulated for each study and end point.
Results: For the PPMI cohort, 417 participants with PD (mean age 61.6 years, 65% male) were followed, with an estimated probability of dementia at year 10 disease duration of 9% (site investigator diagnosis), 15% (MoCA), or 12% (MDS-UPDRS Part I cognition). For the Penn cohort, 389 participants with PD (mean age 69.3 years, 67% male) were followed, with 184 participants (47% of cohort) eventually diagnosed with dementia. The interval-censored curve for the Penn cohort had a median time to dementia of 15 years (95% CI 13-15); the estimated probability of dementia was 27% at 10 years of disease duration, 50% at 15 years, and 74% at 20 years.
Discussion: Results from two large, prospective studies suggest that dementia in PD occurs less frequently, or later in the disease course, than previous research studies have reported.
Conflict of interest statement
C. Gochanour, C. Caspell-Garcia, R.D. Dobkin, L.M. Chahine, C.S. Coffey, A.D. Siderowf, T. Simuni, M.K. York, and D. Weintraub receive financial support for their administrative roles on the PPMI study. R.D. Dobkin receives grant support from the Michael J. Fox Foundation and the VA Office of Rural Health. D. Aarsland has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals, Evonik, Roche Diagnostics, GE Health, and Sanofi, and has received consulting fees from H. Lundbeck, Eisai, Heptares, Mentis Cura, Eli Lilly, Cognetivity, Enterin, Acadia, EIP Pharma, Biogen, and Takeda. R.N. Alcalay has received consultation fees from Capsida, Takeda, Sanofi, Biohaven and Gain Therapeutics (paid to institution), and has received research support from the Parkinson's Foundation, the Michael J. Fox Foundation, and the Silverstein Foundation. M.J. Barrett receives research funding from the NIH (R21AG077469, R21AG074368) and Kyowa Kirin Inc., and serves as site PI for clinical trials and studies sponsored by Biogen, the CHDI Foundation, Cognition Therapeutics, EIP Pharma, uniQure, the Parkinson's Foundation, and Prilenia Therapeutics. L.M. Chahine receives research support from the Michael J. Fox Foundation, the UPMC Competitive Medical Research Fund, NIH, and the University of Pittsburgh, is site investigator for a study sponsored by Biogen, has received consulting fees from the Michael J. Fox Foundation, and receives royalties from Elsevier and Wolters Kluwer (for authorship). A. Chen-Plotkin receives research support from the NIH and the Parker Family Chair, is the inventor of a patent held by the University of Pennsylvania pertaining to genetic approaches to treating frontotemporal dementia (receives royalties for licensing). C.S. Coffey receives grant funding from the Michael J. Fox Foundation and the National Institute for Neurological Disorders and Stroke. N. Dahodwala receives grant support from the NIH (R01NS125294, U19AG062418), the Michael J. Fox Foundation, the Parkinson's Foundation, and Genentech (clinical trial), has received honoraria from Mediflix, has received consulting fees from Genentech, and has received compensation for review of medical records for Post & Schell (law firm). A.J. Espay has received grant support from the NIH and the Michael J. Fox Foundation, has received personal compensation as a consultant/scientific advisory board member for Neuroderm, Amneal, Acadia, Avion Pharmaceuticals, Acorda, Kyowa Kirin, Supernus (formerly, USWorldMeds), and Herantis Pharma, has received speaker honoraria from Avion, Amneal, and Supernus, has received publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer, is a cofounder of REGAIN Therapeutics, and is co-inventor of the patent “Compositions and methods for treatment and/or prophylaxis of proteinopathies.” J.B. Leverenz has received research support from the NIH (P30AG072959, U01NS100610, U01AG073323), GE Healthcare, the Alzheimer's Association, and the Lewy Body Dementia Association. I. Litvan has received research support from the NIH (grants: 2R01AG038791-06A, U01NS100610, R25NS098999, U19 AG063911-1, and 1R21NS114764-01A1), the Michael J. Fox Foundation, the Parkinson's Foundation, the Lewy Body Association, CurePSP, Roche, Abbvie, Biogen, Centogene, EIP-Pharma, Biohaven Pharmaceuticals, Novartis, and United Biopharma SRL UCB, is a member of the scientific advisory board for Amydis (no compensation received) and the Rossy PSP Program at the University of Toronto, and receives salary from the University of California San Diego and as Chief Editor of
Figures
Comment in
-
Demenz bei Parkinson doch nicht so häufig?MMW Fortschr Med. 2024 Dec;166(21-22):31. doi: 10.1007/s15006-024-4551-6. MMW Fortschr Med. 2024. PMID: 39653909 Review. German. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
