Assessing the Short-Term Efficacy of Digital Cognitive Behavioral Therapy for Insomnia With Different Types of Coaching: Randomized Controlled Comparative Trial
- PMID: 39110971
- PMCID: PMC11339566
- DOI: 10.2196/51716
Assessing the Short-Term Efficacy of Digital Cognitive Behavioral Therapy for Insomnia With Different Types of Coaching: Randomized Controlled Comparative Trial
Abstract
Background: Digital cognitive behavioral therapy for insomnia (dCBTi) is an effective intervention for treating insomnia. The findings regarding its efficacy compared to face-to-face cognitive behavioral therapy for insomnia are inconclusive but suggest that dCBTi might be inferior. The lack of human support and low treatment adherence are believed to be barriers to dCBTi achieving its optimal efficacy. However, there has yet to be a direct comparative trial of dCBTi with different types of coaching support.
Objective: This study examines whether adding chatbot-based and human coaching would improve the treatment efficacy of, and adherence to, dCBTi.
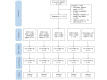
Methods: Overall, 129 participants (n=98, 76% women; age: mean 34.09, SD 12.05 y) whose scores on the Insomnia Severity Index [ISI] were greater than 9 were recruited. A randomized controlled comparative trial with 5 arms was conducted: dCBTi with chatbot-based coaching and therapist support (dCBTi-therapist), dCBTi with chatbot-based coaching and research assistant support, dCBTi with chatbot-based coaching only, dCBTi without any coaching, and digital sleep hygiene and self-monitoring control. Participants were blinded to the condition assignment and study hypotheses, and the outcomes were self-assessed using questionnaires administered on the web. The outcomes included measures of insomnia (the ISI and the Sleep Condition Indicator), mood disturbances, fatigue, daytime sleepiness, quality of life, dysfunctional beliefs about sleep, and sleep-related safety behaviors administered at baseline, after treatment, and at 4-week follow-up. Treatment adherence was measured by the completion of video sessions and sleep diaries. An intention-to-treat analysis was conducted.
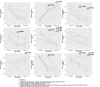
Results: Significant condition-by-time interaction effects showed that dCBTi recipients, regardless of having any coaching, had greater improvements in insomnia measured by the Sleep Condition Indicator (P=.003; d=0.45) but not the ISI (P=.86; d=-0.28), depressive symptoms (P<.001; d=-0.62), anxiety (P=.01; d=-0.40), fatigue (P=.02; d=-0.35), dysfunctional beliefs about sleep (P<.001; d=-0.53), and safety behaviors related to sleep (P=.001; d=-0.50) than those who received digital sleep hygiene and self-monitoring control. The addition of chatbot-based coaching and human support did not improve treatment efficacy. However, adding human support promoted greater reductions in fatigue (P=.03; d=-0.33) and sleep-related safety behaviors (P=.05; d=-0.30) than dCBTi with chatbot-based coaching only at 4-week follow-up. dCBTi-therapist had the highest video and diary completion rates compared to other conditions (video: 16/25, 60% in dCBTi-therapist vs <3/21, <25% in dCBTi without any coaching), indicating greater treatment adherence.
Conclusions: Our findings support the efficacy of dCBTi in treating insomnia, reducing thoughts and behaviors that perpetuate insomnia, reducing mood disturbances and fatigue, and improving quality of life. Adding chatbot-based coaching and human support did not significantly improve the efficacy of dCBTi after treatment. However, adding human support had incremental benefits on reducing fatigue and behaviors that could perpetuate insomnia, and hence may improve long-term efficacy.
Trial registration: ClinicalTrials.gov NCT05136638; https://www.clinicaltrials.gov/study/NCT05136638.
Keywords: chatbot-based coaching; cognitive behavioral therapy; digital intervention; human support; insomnia; mHealth; mobile health; mobile phone.
©Wai Sze Chan, Wing Yee Cheng, Samson Hoi Chun Lok, Amanda Kah Mun Cheah, Anna Kai Win Lee, Albe Sin Ying Ng, Tobias Kowatsch. Originally published in JMIR Mental Health (https://mental.jmir.org), 07.08.2024.
Conflict of interest statement
Conflicts of Interest: TK is affiliated with the Centre for Digital Health Interventions, a joint initiative of the Institute for Implementation Science in Health Care at the University of Zurich; the Department of Management, Technology, and Economics at ETH Zurich; the Future Health Technologies Program at the Singapore-ETH Centre; and the School of Medicine and Institute of Technology Management at the University of St Gallen. The Centre for Digital Health Interventions is funded in part by CSS, a Swiss health insurer; Mavie Next (owned by the UNIQA Group), an Austrian care provider; and MTIP, a Swiss investor company. TK is also a cofounder of Pathmate Technologies, a university spin-off company that creates and delivers digital clinical pathways. However, CSS, Pathmate Technologies, Mavie Next, and MTIP were not involved in this research. All other authors declare no other conflicts of interest.
Figures
Similar articles
-
Effect of Digital Cognitive Behavioral Therapy for Insomnia on Health, Psychological Well-being, and Sleep-Related Quality of Life: A Randomized Clinical Trial.JAMA Psychiatry. 2019 Jan 1;76(1):21-30. doi: 10.1001/jamapsychiatry.2018.2745. JAMA Psychiatry. 2019. PMID: 30264137 Free PMC article. Clinical Trial.
-
Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial.Sleep. 2014 Aug 1;37(8):1305-14. doi: 10.5665/sleep.3918. Sleep. 2014. PMID: 25083010 Free PMC article. Clinical Trial.
-
Sleep Treatment Education Program for Cancer Survivors: Protocol for an Efficacy Trial.JMIR Res Protoc. 2024 Nov 28;13:e60762. doi: 10.2196/60762. JMIR Res Protoc. 2024. PMID: 39608001 Free PMC article.
-
Is cognitive behavioral therapy for insomnia more cost-effective? New-perspective on economic evaluations: a systematic review and meta-analysis.Sleep. 2024 Aug 14;47(8):zsae122. doi: 10.1093/sleep/zsae122. Sleep. 2024. PMID: 38795362
-
Effects of Digital Sleep Interventions on Sleep Among College Students and Young Adults: Systematic Review and Meta-Analysis.J Med Internet Res. 2025 May 12;27:e69657. doi: 10.2196/69657. J Med Internet Res. 2025. PMID: 40354636 Free PMC article. Review.
Cited by
-
Toward a Smartphone-Based and Conversational Agent-Delivered Just-in-Time Adaptive Holistic Lifestyle Intervention for Older Adults Affected by Cognitive Decline: Two-Week Proof-of-Concept Study.JMIR Form Res. 2025 Jul 28;9:e66885. doi: 10.2196/66885. JMIR Form Res. 2025. PMID: 40720890 Free PMC article.
-
Who is the "Therapist" in Digital Cognitive Behavioural Therapy for Insomnia (dCBTi)?Nat Sci Sleep. 2025 Jun 13;17:1319-1324. doi: 10.2147/NSS.S516276. eCollection 2025. Nat Sci Sleep. 2025. PMID: 40535057 Free PMC article.
-
Sleep habits in the pathogenesis and management of diabesity.J Diabetes Investig. 2025 Jul;16(7):1202-1216. doi: 10.1111/jdi.70075. Epub 2025 Jun 1. J Diabetes Investig. 2025. PMID: 40452148 Free PMC article. Review.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5) Washington, DC: American Psychiatric Association Publishing; 2013.
-
- The AASM International Classification of Sleep Disorders – Third Edition, Text Revision (ICSD-3-TR) American Academy of Sleep Medicine. [2024-06-17]. https://aasm.org/clinical-resources/international-classification-sleep-d...
-
- Lam CS, Yu BY, Cheung DS, Cheung T, Lam SC, Chung KF, Ho FY, Yeung WF. Sleep and mood disturbances during the COVID-19 outbreak in an urban Chinese population in Hong Kong: a longitudinal study of the second and third waves of the outbreak. Int J Environ Res Public Health. 2021 Aug 10;18(16):8444. doi: 10.3390/ijerph18168444. https://www.mdpi.com/resolver?pii=ijerph18168444 ijerph18168444 - DOI - PMC - PubMed
-
- Morin CM, Bjorvatn B, Chung F, Holzinger B, Partinen M, Penzel T, Ivers H, Wing YK, Chan NY, Merikanto I, Mota-Rolim S, Macêdo T, De Gennaro L, Léger D, Dauvilliers Y, Plazzi G, Nadorff MR, Bolstad CJ, Sieminski M, Benedict C, Cedernaes J, Inoue Y, Han F, Espie CA. Insomnia, anxiety, and depression during the COVID-19 pandemic: an international collaborative study. Sleep Med. 2021 Nov;87:38–45. doi: 10.1016/j.sleep.2021.07.035. https://europepmc.org/abstract/MED/34508986 S1389-9457(21)00419-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

