SLC25A48 controls mitochondrial choline import and metabolism
- PMID: 39111307
- PMCID: PMC11953726
- DOI: 10.1016/j.cmet.2024.07.010
SLC25A48 controls mitochondrial choline import and metabolism
Abstract
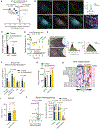
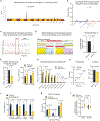
Choline is an essential nutrient for the biosynthesis of phospholipids, neurotransmitters, and one-carbon metabolism with a critical step being its import into mitochondria. However, the underlying mechanisms and biological significance remain poorly understood. Here, we report that SLC25A48, a previously uncharacterized mitochondrial inner-membrane carrier protein, controls mitochondrial choline transport and the synthesis of choline-derived methyl donors. We found that SLC25A48 was required for brown fat thermogenesis, mitochondrial respiration, and mitochondrial membrane integrity. Choline uptake into the mitochondrial matrix via SLC25A48 facilitated the synthesis of betaine and purine nucleotides, whereas loss of SLC25A48 resulted in increased production of mitochondrial reactive oxygen species and imbalanced mitochondrial lipids. Notably, human cells carrying a single nucleotide polymorphism on the SLC25A48 gene and cancer cells lacking SLC25A48 exhibited decreased mitochondrial choline import, increased oxidative stress, and impaired cell proliferation. Together, this study demonstrates that SLC25A48 regulates mitochondrial choline catabolism, bioenergetics, and cell survival.
Keywords: bioenergetics; brown adipose tissue; cancer metabolism; choline; mitochondria; purine nucleotides.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
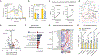

Update of
-
Mitochondrial choline import regulates purine nucleotide pools via SLC25A48.bioRxiv [Preprint]. 2024 Jan 1:2023.12.31.573776. doi: 10.1101/2023.12.31.573776. bioRxiv. 2024. Update in: Cell Metab. 2024 Sep 3;36(9):2156-2166.e9. doi: 10.1016/j.cmet.2024.07.010. PMID: 38260464 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

