Male autism spectrum disorder is linked to brain aromatase disruption by prenatal BPA in multimodal investigations and 10HDA ameliorates the related mouse phenotype
- PMID: 39112449
- PMCID: PMC11306638
- DOI: 10.1038/s41467-024-48897-8
Male autism spectrum disorder is linked to brain aromatase disruption by prenatal BPA in multimodal investigations and 10HDA ameliorates the related mouse phenotype
Abstract
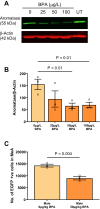
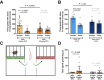
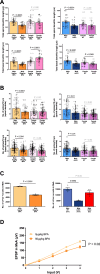
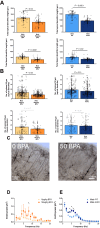
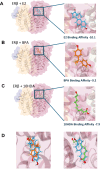
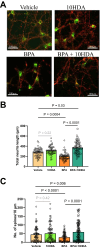
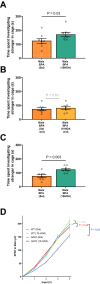
Male sex, early life chemical exposure and the brain aromatase enzyme have been implicated in autism spectrum disorder (ASD). In the Barwon Infant Study birth cohort (n = 1074), higher prenatal maternal bisphenol A (BPA) levels are associated with higher ASD symptoms at age 2 and diagnosis at age 9 only in males with low aromatase genetic pathway activity scores. Higher prenatal BPA levels are predictive of higher cord blood methylation across the CYP19A1 brain promoter I.f region (P = 0.009) and aromatase gene methylation mediates (P = 0.01) the link between higher prenatal BPA and brain-derived neurotrophic factor methylation, with independent cohort replication. BPA suppressed aromatase expression in vitro and in vivo. Male mice exposed to mid-gestation BPA or with aromatase knockout have ASD-like behaviors with structural and functional brain changes. 10-hydroxy-2-decenoic acid (10HDA), an estrogenic fatty acid alleviated these features and reversed detrimental neurodevelopmental gene expression. Here we demonstrate that prenatal BPA exposure is associated with impaired brain aromatase function and ASD-related behaviors and brain abnormalities in males that may be reversible through postnatal 10HDA intervention.
© 2024. The Author(s).
Conflict of interest statement
W.C.B. is a co-inventor on ‘Methods of treating neurodevelopmental diseases and disorders’, USA Patent No. US9925163B2, Australian Patent No. 2015271652. This has been licensed to Meizon Innovation Holdings. A.-L.P. is a scientific advisor and W.C.B. is a board member of the Meizon Innovation Holdings. The remaining authors declare no competing interests.
Figures

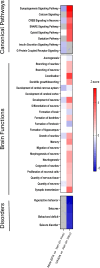
References
-
- American Psychiatric Association aAPAD-TF. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (American Psychiatric Association, 2013).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

