Sacituzumab Govitecan in patients with breast cancer brain metastases and recurrent glioblastoma: a phase 0 window-of-opportunity trial
- PMID: 39112464
- PMCID: PMC11306739
- DOI: 10.1038/s41467-024-50558-9
Sacituzumab Govitecan in patients with breast cancer brain metastases and recurrent glioblastoma: a phase 0 window-of-opportunity trial
Abstract
Sacituzumab Govitecan (SG) is an antibody-drug conjugate that has demonstrated efficacy in patients with TROP-2 expressing epithelial cancers. In a xenograft model of intracranial breast cancer, SG inhibited tumor growth and increased mouse survival. We conducted a prospective window-of-opportunity trial (NCT03995706) at the University of Texas Health Science Center at San Antonio to examine the intra-tumoral concentrations and intracranial activity of SG in patients undergoing craniotomy for breast cancer with brain metastases (BCBM) or recurrent glioblastoma (rGBM). We enrolled 25 patients aged ≥18 years diagnosed with BCBM and rGBM to receive a single intravenous dose of SG at 10 mg/kg given one day before resection and continued on days 1 and 8 of 21-day cycles following recovery. The PFS was 8 months and 2 months for BCBM and rGBM cohorts, respectively. The OS was 35.2 months and 9.5 months, respectively. Grade≥3 AE included neutropenia (28%), hypokalemia (8%), seizure (8%), thromboembolic event (8%), urinary tract infection (8%) and muscle weakness of the lower limb (8%). In post-surgical tissue, the median total SN-38 was 249.8 ng/g for BCBM and 104.5 ng/g for rGBM, thus fulfilling the primary endpoint. Biomarker analysis suggests delivery of payload by direct release at target site and that hypoxic changes do not drive indirect release. Secondary endpoint of OS was 35.2 months for the BCBM cohort and 9.5 months for rGBM. Non-planned exploratory endpoint of ORR was 38% for BCBM and 29%, respectively. Exploratory endpoint of Trop-2 expression was observed in 100% of BCBM and 78% of rGBM tumors. In conclusion, SG was found to be well tolerated with adequate penetration into intracranial tumors and promising preliminary activity within the CNS. Trial Registration: Trial (NCT03995706) enrolled at Clinical Trials.gov as Neuro/Sacituzumab Govitecan/Breast Brain Metastasis/Glioblastoma/Ph 0: https://clinicaltrials.gov/study/NCT03995706?cond=NCT03995706 .
© 2024. The Author(s).
Conflict of interest statement
HB, WK, KL, PG, GS, JM, AG, PS, ST, RP, JC, AL, JF and AB have no personal, professional or financial relationships that would constitute a conflict of interest. VK and MC are on the speaker’s bureau and VK is a consultant for Gilead. Portions of this work have previously been presented in abstract form at the 2023 Society for NeuroOncology Annual Meeting, San Antonio Breast Cancer Symposium 2022 and 2024, and the European Society for Medical Oncology Virtual Congress 2020. WK and AB have received contracted funding from Gilead for a similar study.
Figures
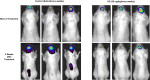
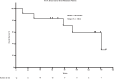



References
-
- Montemurro, F. et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial☆. Ann. Oncol.31, 1350–1358 (2020). 10.1016/j.annonc.2020.06.020 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

