Neuronal substance P drives metastasis through an extracellular RNA-TLR7 axis
- PMID: 39112700
- PMCID: PMC11633843
- DOI: 10.1038/s41586-024-07767-5
Neuronal substance P drives metastasis through an extracellular RNA-TLR7 axis
Abstract
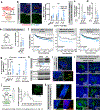
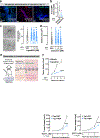
Tumour innervation is associated with worse patient outcomes in multiple cancers1,2, which suggests that it may regulate metastasis. Here we observed that highly metastatic mouse mammary tumours acquired more innervation than did less-metastatic tumours. This enhanced innervation was driven by expression of the axon-guidance molecule SLIT2 in tumour vasculature. Breast cancer cells induced spontaneous calcium activity in sensory neurons and elicited release of the neuropeptide substance P (SP). Using three-dimensional co-cultures and in vivo models, we found that neuronal SP promoted breast tumour growth, invasion and metastasis. Moreover, patient tumours with elevated SP exhibited enhanced lymph node metastatic spread. SP acted on tumoral tachykinin receptors (TACR1) to drive death of a small population of TACR1high cancer cells. Single-stranded RNAs (ssRNAs) released from dying cells acted on neighbouring tumoural Toll-like receptor 7 (TLR7) to non-canonically activate a prometastatic gene expression program. This SP- and ssRNA-induced Tlr7 gene expression signature was associated with reduced breast cancer survival outcomes. Therapeutic targeting of this neuro-cancer axis with the TACR1 antagonist aprepitant, an approved anti-nausea drug, suppressed breast cancer growth and metastasis in multiple models. Our findings reveal that tumour-induced hyperactivation of sensory neurons regulates multiple aspects of metastatic progression in breast cancer through a therapeutically targetable neuropeptide/extracellular ssRNA sensing axis.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures










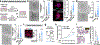


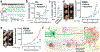
Comment in
-
Neurons give metastatic cells a push.Nat Rev Drug Discov. 2024 Oct;23(10):739. doi: 10.1038/d41573-024-00144-x. Nat Rev Drug Discov. 2024. PMID: 39251736 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

