DNA-sensing inflammasomes cause recurrent atherosclerotic stroke
- PMID: 39112714
- PMCID: PMC11390481
- DOI: 10.1038/s41586-024-07803-4
DNA-sensing inflammasomes cause recurrent atherosclerotic stroke
Abstract
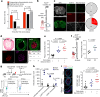
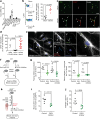
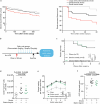
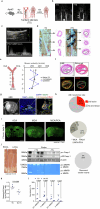
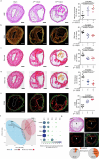
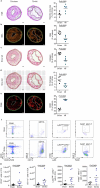
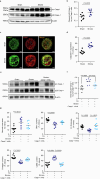
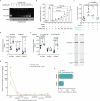
The risk of early recurrent events after stroke remains high despite currently established secondary prevention strategies1. Risk is particularly high in patients with atherosclerosis, with more than 10% of patients experiencing early recurrent events1,2. However, despite the enormous medical burden of this clinical phenomenon, the underlying mechanisms leading to increased vascular risk and recurrent stroke are largely unknown. Here, using a novel mouse model of stroke-induced recurrent ischaemia, we show that stroke leads to activation of the AIM2 inflammasome in vulnerable atherosclerotic plaques via an increase of circulating cell-free DNA. Enhanced plaque inflammation post-stroke results in plaque destabilization and atherothrombosis, finally leading to arterioarterial embolism and recurrent stroke within days after the index stroke. We confirm key steps of plaque destabilization also after experimental myocardial infarction and in carotid artery plaque samples from patients with acute stroke. Rapid neutrophil NETosis was identified as the main source of cell-free DNA after stroke and NET-DNA as the causative agent leading to AIM2 inflammasome activation. Neutralization of cell-free DNA by DNase treatment or inhibition of inflammasome activation reduced the rate of stroke recurrence after experimental stroke. Our findings present an explanation for the high recurrence rate after incident ischaemic events in patients with atherosclerosis. The detailed mechanisms uncovered here provide clinically uncharted therapeutic targets for which we show high efficacy to prevent recurrent events. Targeting DNA-mediated inflammasome activation after remote tissue injury represents a promising avenue for further clinical development in the prevention of early recurrent events.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Comment in
-
Degrading cell-free DNA to prevent recurrent stroke.Nat Rev Drug Discov. 2024 Oct;23(10):740. doi: 10.1038/d41573-024-00136-x. Nat Rev Drug Discov. 2024. PMID: 39174640 No abstract available.
References
-
- World Health Organization. The top 10 causes of death. WHOhttps://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

