AI-based automation of enrollment criteria and endpoint assessment in clinical trials in liver diseases
- PMID: 39112795
- PMCID: PMC11485234
- DOI: 10.1038/s41591-024-03172-7
AI-based automation of enrollment criteria and endpoint assessment in clinical trials in liver diseases
Abstract
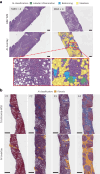
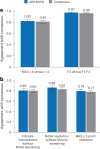
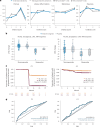
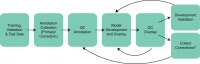
Clinical trials in metabolic dysfunction-associated steatohepatitis (MASH, formerly known as nonalcoholic steatohepatitis) require histologic scoring for assessment of inclusion criteria and endpoints. However, variability in interpretation has impacted clinical trial outcomes. We developed an artificial intelligence-based measurement (AIM) tool for scoring MASH histology (AIM-MASH). AIM-MASH predictions for MASH Clinical Research Network necroinflammation grades and fibrosis stages were reproducible (κ = 1) and aligned with expert pathologist consensus scores (κ = 0.62-0.74). The AIM-MASH versus consensus agreements were comparable to average pathologists for MASH Clinical Research Network scores (82% versus 81%) and fibrosis (97% versus 96%). Continuous scores produced by AIM-MASH for key histological features of MASH correlated with mean pathologist scores and noninvasive biomarkers and strongly predicted progression-free survival in patients with stage 3 (P < 0.0001) and stage 4 (P = 0.03) fibrosis. In a retrospective analysis of the ATLAS trial (NCT03449446), responders receiving study treatment showed a greater continuous change in fibrosis compared with placebo (P = 0.02). Overall, these results suggest that AIM-MASH may assist pathologists in histologic review of MASH clinical trials, reducing inter-rater variability on trial outcomes and offering a more sensitive and reproducible measure of patient responses.
© 2024. The Author(s).
Conflict of interest statement
A.N.B. is an employee of and holds stock in Gilead Sciences, Inc., and received study materials from PathAI, Inc. in support of this manuscript. A.D.B. serves as a consultant to 23andMe, Alimentiv, Allergan, Dialectica, PathAI, Inc., Source Bioscience and Verily, and is on Scientific Advisory Boards with 3Helix, Avacta and GSK. His institution has received funding for educational programs from Eli Lilly. A.H.B. is an employee of and holds stock in PathAI, Inc. A.P. is a former employee of, holds stock in and owns patents with PathAI, Inc. A.T.-W. is a former employee of and owns stock in PathAI, Inc. C.B.-S. is a former employee of and holds stock in PathAI, Inc. C.C. is an employee of Inipharm, a former employee of Gilead Sciences, Inc., and owns stock in Gilead Sciences, Inc. and Inipharm. D.J. is an employee of, holds stock in and owns patents with PathAI, Inc. H.E. is a former employee of and holds stock in PathAI, Inc., and is named on a patent (US 11527319) held by PathAI, Inc. H.P. is an employee of, owns stock in and owns patents with PathAI, Inc. I.W. is a former employee of and owns stock in PathAI, Inc., and owns a patent (US 10650520). J.G. is a former employee of and owns stock in PathAI, Inc., J.S.I. is a former employee of and owns stock in PathAI, Inc., and owns a patent. K.L. is a former employee of and owns stock in PathAI, Inc. and received an ISO grant while employed at PathAI, Inc. K.W. is a former employee of, owns stock in, received support for meeting attendance from and receives consulting fees from PathAI, Inc. M.L. is a former employee of and owns stock in PathAI, Inc. M.C.M. is a former employee of, holds stock in and receives financial support to attend meetings from PathAI, Inc.; holds stock in Bristol Myers Squibb; and holds a leadership position with the Digital Pathology Association. M.R. is a former employee of, owns stock in and receives consulting fees from PathAI, Inc. M.P. is a former employee of and holds stock in PathAI, Inc. O.C.-Z. is a former employee of and holds stock options in PathAI, Inc., and has a patent pending (US 20220245802A1). Q.L. is an employee of and owns stock in PathAI, Inc., and owns a patent. R.L. serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, Astra Zeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead Sciences, Inc., Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead Sciences, Inc., Intercept, Hanmi, Inventiva, Ionis, Janssen, Inc., Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Pfizer, Sonic Incytes and Terns Pharmaceuticals. He is a co-founder of LipoNexus, Inc. R.P.M. is an employee of OrsoBio, Inc., and owns stock in OrsoBio, Inc. and Gilead Sciences, Inc. S.A.S.-M. is an employee of and owns stock in PathAI, Inc. R.E. is an employee of and owns stock in PathAI, Inc. S.H. is a former employee of, owns stock in and received support for meeting attendance from PathAI, Inc. S.D.P. is an employee of and holds stock in Gilead Sciences. T.R.W. is an employee of and holds stock in Gilead Sciences, Inc. Z.S. is an employee of and holds stock in PathAI, Inc., and owns a patent with PathAI, Inc. B.G. is an employee of, holds stock in and receives support for meeting attendance from PathAI, Inc. A.J.S. holds stock options in Genfit, Akarna, Tiziana, Durect, Inversago, Hemoshear, Northsea, Diapin, Liponexus and Galmed. In addition, he serves as a consultant to Astra Zeneca (<5 K), Terns (<5 K), Merck (<5 K), Boehringer Ingelheim (5–10 K), Lilly (5–10 K), Novartis (<5 K), Novo Nordisk (<5 K), Pfizer (<5 K), 89 Bio (<5 K), Regeneron (<5 K), Alnylam (<5 K), Akero (<5 K), Tern (<5 K), Histoindex (<5 K), Corcept (<5 K), PathAI (<5 K), Genfit (<5 K), Mediar (<5 K), Satellite Bio (<5 K), Echosens (<5 K), Abbott (<5 K), Promed (<5 K), Glaxo Smith Kline (∼11 K), Arrowhead (<5 K), Zydus (>60 K), Boston Pharmaceutical (<5 K), Myovent (<5 K), Variant (<5 K), Cascade (<5 K) and Northsea (<5 K), and his institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Mallinckrodt and Novartis. Lastly, he receives royalties from Elsevier and UpToDate.
Figures





References
-
- Younossi, Z. M. et al. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology69, 564–572 (2019). - PubMed
-
- Kingwell, K. NASH field celebrates ‘hurrah moment’ with a first FDA drug approval for the liver disease. Nat. Rev. Drug Discov.23, 235–237 (2024). - PubMed
-
- Naoumov, N. V. et al. Digital pathology with artificial intelligence analyses provides greater insights into treatment-induced fibrosis regression in NASH. J. Hepatol.77, 1399–1409 (2022). - PubMed

