RECIST 1.1 assessments variability: a systematic pictorial review of blinded double reads
- PMID: 39112819
- PMCID: PMC11306910
- DOI: 10.1186/s13244-024-01774-w
RECIST 1.1 assessments variability: a systematic pictorial review of blinded double reads
Abstract
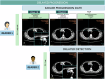
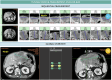
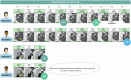
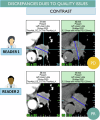
Reader variability is intrinsic to radiologic oncology assessments, necessitating measures to enhance consistency and accuracy. RECIST 1.1 criteria play a crucial role in mitigating this variability by standardizing evaluations, aiming to establish an accepted "truth" confirmed by histology or patient survival. Clinical trials utilize Blind Independent Centralized Review (BICR) techniques to manage variability, employing double reads and adjudicators to address inter-observer discordance effectively. It is essential to dissect the root causes of variability in response assessments, with a specific focus on the factors influencing RECIST evaluations. We propose proactive measures for radiologists to address variability sources such as radiologist expertise, image quality, and accessibility of contextual information, which significantly impact interpretation and assessment precision. Adherence to standardization and RECIST guidelines is pivotal in diminishing variability and ensuring uniform results across studies. Variability factors, including lesion selection, new lesion appearance, and confirmation bias, can have profound implications on assessment accuracy and interpretation, underscoring the importance of identifying and addressing these factors. Delving into the causes of variability aids in enhancing the accuracy and consistency of response assessments in oncology, underscoring the role of standardized evaluation protocols and mitigating risk factors that contribute to variability. Access to contextual information is crucial. CRITICAL RELEVANCE STATEMENT: By understanding the causes of diagnosis variability, we can enhance the accuracy and consistency of response assessments in oncology, ultimately improving patient care and clinical outcomes. KEY POINTS: Baseline lesion selection and detection of new lesions play a major role in the occurrence of discordance. Image interpretation is influenced by contextual information, the lack of which can lead to diagnostic uncertainty. Radiologists must be trained in RECIST criteria to reduce errors and variability.
Keywords: Diagnostic errors; Oncology; Quality improvement; RECIST 1.1; Statistics & numerical data.
© 2024. The Author(s).
Conflict of interest statement
Antoine Iannessi & Hubert Beaumont are part-time employees of Median Technologies. Christine Ojango & Yan Liu are full-time employees of Median Technologies. Anne-Sophie Bertrand declares having no competing interests.
Figures
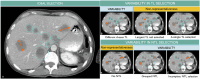
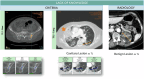
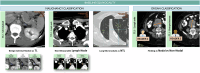
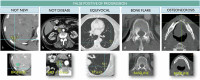




References
-
- (FDA) FaDA (2018) Clinical trial imaging endpoint process standards guidance for industry. In: Research CfDEaRCfBEa (ed.). FDA. 26 Apr 2018
-
- Ford R, O’Neal M, Moskowitz S, Fraunberger J (2016) Adjudication rates between readers in Blinded Independent Central Review of Oncology Studies. J Clin Trials 6:289
Publication types
LinkOut - more resources
Full Text Sources

