Highlights of Gene and Cell Therapy for Epidermolysis Bullosa and Ichthyosis
- PMID: 39112824
- PMCID: PMC11393223
- DOI: 10.1007/s13555-024-01239-4
Highlights of Gene and Cell Therapy for Epidermolysis Bullosa and Ichthyosis
Abstract
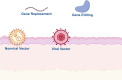
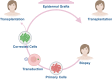
Advancements in the molecular genetics of epidermolysis bullosa (EB) and ichthyosis, two rare inherited skin conditions, have enabled the identification of genetic variants that cause these diseases. Alongside technological advancements in genetic medicine, the identification of variants causal of these rare skin conditions has led to preclinical research and the clinical development of various in vivo and ex vivo gene and cell therapies for their treatment. Gene and cell therapies are considered to be the most advanced forms of personalized medicine, demonstrating safety and efficacy in numerous rare diseases. Although the orphan drug development boom has resulted in regulatory approval of multiple gene and cell therapies for various rare conditions, the application of these modalities to rare inherited skin conditions remains limited. Nonetheless, there are successful examples of both in vivo gene therapy- and ex vivo cell therapy-based approaches developed to treat EB and ichthyosis. This review highlights preclinical research and the clinical development of gene and cell therapies for multiple subtypes of these two devastating congenital skin conditions, including a gene therapy recently approved by the U.S. Food and Drug Administration for the treatment of recessive dystrophic EB.
Keywords: Cell therapy; Epidermolysis bullosa; Gene therapy; Ichthyosis; Rare disease.
Plain language summary
Advances in genetics research for skin diseases such as epidermolysis bullosa and ichthyosis have led to the discovery of many new subtypes of these severe skin conditions. Identifying new subtypes has in turn led to new treatments for these conditions, including gene and cell therapies. Gene and cell therapies aim to address the underlying genetic causes of disease and have already shown success in the clinic. While the development of such treatments for rare skin diseases has been limited, there are notable examples of gene and cell therapies developed for epidermolysis bullosa and ichthyosis. This review highlights recent developments in gene and cell therapy for epidermolysis bullosa and ichthyosis, including a newly approved gene therapy for recessive dystrophic epidermolysis bullosa.
© 2024. The Author(s).
Conflict of interest statement
Stefanos A. Koutsoukos and Ganna Bilousova declare that they have no competing interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

