Comparison of Different Doses of Oral and Ocular Propranolol for Retinopathy of Prematurity: A Network Meta-Analysis
- PMID: 39112827
- PMCID: PMC11335795
- DOI: 10.1007/s40272-024-00647-5
Comparison of Different Doses of Oral and Ocular Propranolol for Retinopathy of Prematurity: A Network Meta-Analysis
Abstract
Objective: The efficacy and safety of propranolol for retinopathy of prematurity (ROP) remain under debate. This network meta-analysis (NMA) focuses on whether a ranking may be established for different dose levels of propranolol as treatment of ROP in terms of stage progression as the primary outcome, with appearance of plus disease and need for anti-vascular endothelial growth factors (anti-VEGFs) or laser therapy as secondary endpoints.
Methods: Fourteen studies (10 randomised controlled trials, three single-arm trials and one retrospective observational study) of 474 patients treated with oral or ocular propranolol were retrieved from databases up to April 2024. Meta-insight and model-based NMA were undertaken to evaluate the propranolol dose-response relationship. Studies were evaluated for model fit, risk of bias and Confidence of evidence In Network Meta-Analysis (CINeMA). Effect sizes were determined as odds ratio (OR) with 95% credible interval (CrI).
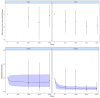
Results: Bayesian analysis showed a trend towards improved effects for propranolol given at late stages (stages 2-3; S23) of ROP progression compared with its administration at earlier stages (stages 0-1; S01). OR values for oral propranolol 1.5 and 2 mg/kg/day given at S23 were 0.13 (95% CrI 0.04-0.37) and 0.16 (95% CrI 0.04-0.61), respectively, while given at S01 were 0.28 (95% CrI 0.02-2.96) and 0.78 (95% CrI 0.14-4.43), respectively. Similarly, OR of eye propranolol 0.2% at S23 was 0.37 (95% CrI 0.09-1.00) versus an S01 OR of 0.64 (95% CrI 0.21-2.04). Surface under the cumulative ranking curve (SUCRA) analyses confirmed best probability values for oral propranolol 1.5-2 mg/kg followed by eye propranolol 0.2%, all at S23. Model-based NMA showed nonlinearity in the dose-response for oral propranolol with a trend to greater maximal effect for its administration at late versus early stages. For secondary endpoints, lower risk values were found with oral propranolol 1.5 mg/kg/day at S23 for progression to plus disease (OR 0.14; 95% CrI 0.02-0.84) and need for anti-VEGFs (OR 0.23; 95% CrI 0.05-0.93) and laser (OR 0.16; 95% CrI 0.02-1.10) therapies also followed by eye propranolol 0.2%, and a similar profile was obtained with SUCRA analysis. Lower doses (0.5-1.0 mg/kg/day) of oral propranolol retained efficacy. Threat of adverse events was estimated as risk difference versus control with no difference for eye propranolol 0.2% and oral propranolol 0.5 mg/kg/day, modest increases of risk for oral propranolol 1.0 and 1.5 mg/kg/day and the highest risk difference for oral propranolol 2.0 mg/kg/day (0.06; 95% CI -0.01 to 0.13).
Conclusion: A diminished risk of disease progression and need for additional treatment was obtained with propranolol in ROP, but safety is a potential concern. Propranolol eye micro-drops (0.2%) can be as efficacious as oral propranolol. Nonetheless, the evidence is limited due to the paucity and quality of the available studies.
© 2024. The Author(s).
Conflict of interest statement
The authors (AO, PM, PR, EM, JLO) declare no financial or non-financial interests that are directly or indirectly related to this work.
Figures




References
-
- Scarboro SD, Harper CA 3rd, Karsaliya G, Ghoraba H, Moshfeghi DM, Al-Khersan H, Robles-Holmes HK, Fan KC, Berrocal AM, Hoyek S, Patel NA, Sbrocca R, Capone A Jr, Drenser KA, Wood EH. The incidence and timing of treatment-requiring retinopathy of prematurity in nanopremature and micropremature infants in the United States: a national multicenter retrospective cohort study. Ophthalmol Retina. 2024;8(3):279–87. 10.1016/j.oret.2023.10.003. 10.1016/j.oret.2023.10.003 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

