One-year outcomes of a novel venous stent for symptomatic iliofemoral venous obstruction: prospective cohort study
- PMID: 39113028
- PMCID: PMC11304576
- DOI: 10.1186/s12916-024-03545-2
One-year outcomes of a novel venous stent for symptomatic iliofemoral venous obstruction: prospective cohort study
Abstract
Background: A stent with characteristics of a hybrid design may have advantages in improving the patency of symptomatic iliofemoral vein obstruction. This study assessed the safety and effectiveness of the V-Mixtent Venous Stent in treating symptomatic iliofemoral outflow obstruction.
Methods: Eligible patients had a Clinical-Etiologic-Anatomic-Physiologic (CEAP) C classification of ≥ 3 or a Venous Clinical Severity Score (VCSS) pain score of ≥ 2. The primary safety endpoint was the rate of major adverse events within 30 days. The primary effectiveness endpoint was the 12-month primary patency rate. Secondary endpoints included changes in VCSS from baseline to 6 and 12 months, alterations in CEAP C classification, Chronic Venous Disease Quality of Life Questionnaire (CIVIQ-14) scores at 12 months, and stent durability measures.
Results: Between December 2020 and November 2021, 171 patients were enrolled across 15 institutions. A total of 185 endovenous stents were placed, with 91.81% of subjects receiving one stent and 8.19% receiving 2 stents. Within 30 days, only two major adverse events occurred (1.17%; 95% confidence interval [CI], 0.14-4.16%), below the literature-defined performance goal of 11% (P < .001). The 12-month primary patency rate (91.36%; 95% CI, 85.93-95.19%; P < .001) exceeded the literature-defined performance goal. VCSS changes from baseline demonstrated clinical improvement at 6 months (- 4.30 ± 3.66) and 12 months (- 4.98 ± 3.67) (P < .001). Significant reduction in symptoms, as measured by CEAP C classification and CIVIQ-14, was observed from pre-procedure to 12 months (P < .001).
Conclusions: The 12-month outcomes confirm the safety and effectiveness of the V-Mixtent Venous Stent in managing symptomatic iliofemoral venous outflow obstruction, including clinical symptom improvement compared to before treatment.
Keywords: Clinical improvement; Iliofemoral venous outflow obstruction; Multicenter study; Patency; Venous stenting.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

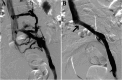
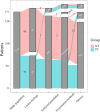
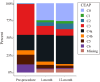
References
-
- Alemany J, Montag H, Wozniak G. Indications for stent implantation in stenoses of the pelvic veins: early results. Vasa Suppl. 1991;33:103. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

